Label: TUSSIN EXPECTORANT- guaifenesin liquid
- NDC Code(s): 76281-507-24
- Packager: AptaPharma, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
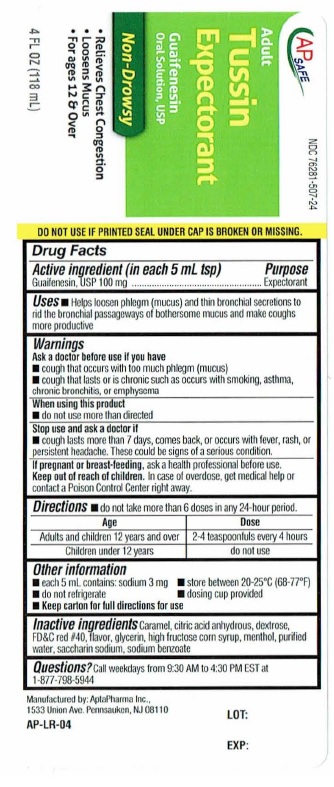
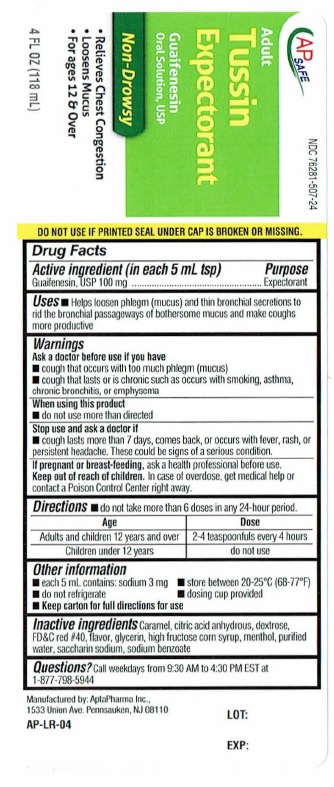
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
-
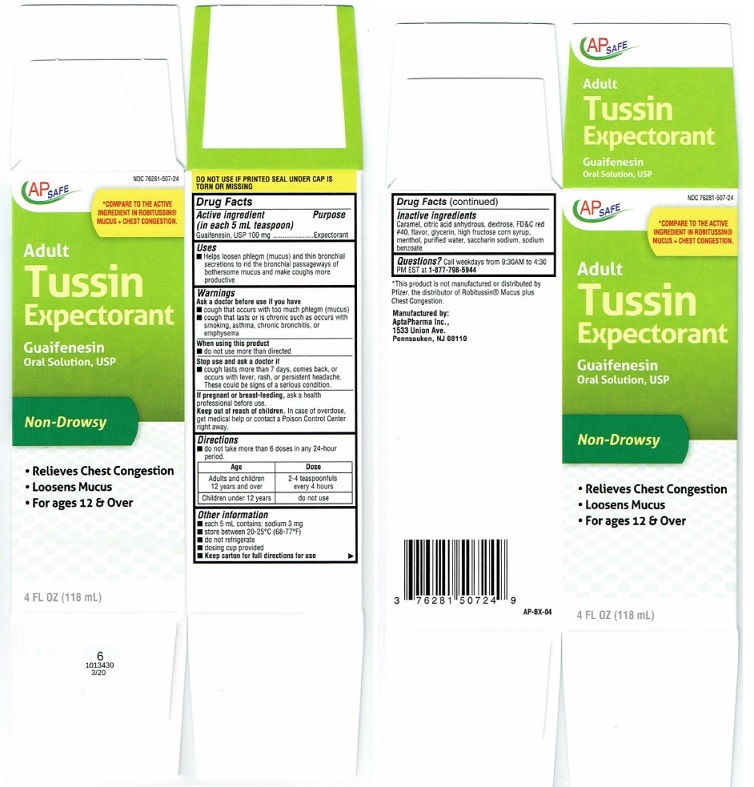
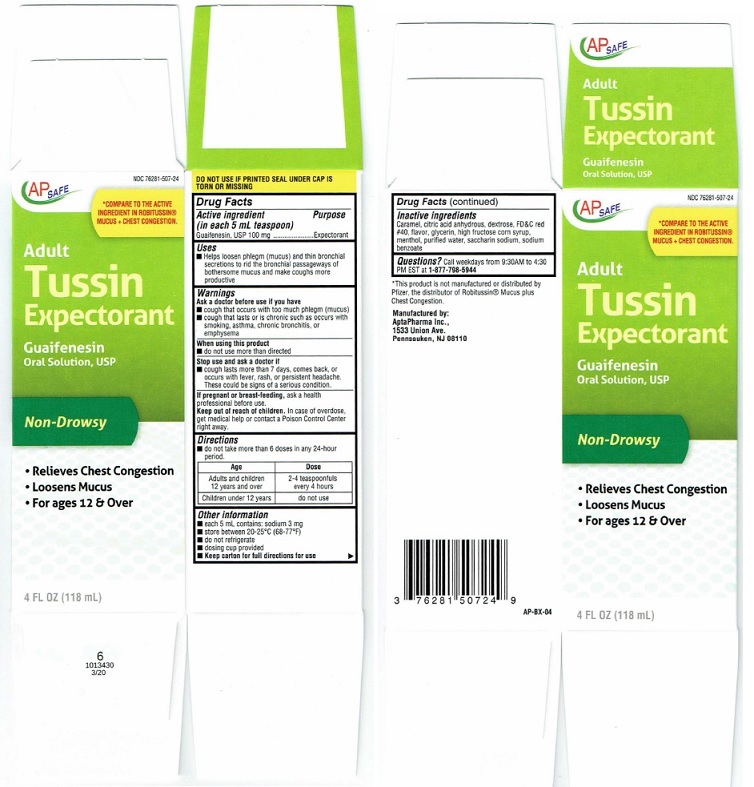
Package Label
AP SAFE NDC 76281-507-24
*COMPARE TO THE ACTIVE
INGREDIENT IN ROBITUSSIN®
MUCUS+CHEST CONGESTION.Adult
Tussin
ExpectorantGuaifenesin
Oral Solution, USP
Non-Drowsy• Relieves Chest Congestion
• Loosens Mucus
• For ages 12 & Over4 FL OZ (118 mL)
DO NOT USE IF PRINTED SEAL UNDER CAP IS
TORN OR MISSINGAP-BX-04
*This product is not manutactured or distributed by
Pfizer, the distributor of Robitussin® Mucus plus
Chest Congestion.Manufactured by:
AplaPharma Inc.,
1533 Union Ave.
Pennsauken, NJ 08110Carton
Bottle
res
-
INGREDIENTS AND APPEARANCE
TUSSIN EXPECTORANT
guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76281-507 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength CARAMEL (UNII: T9D99G2B1R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) DEXTROSE (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76281-507-24 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 09/30/2020 Labeler - AptaPharma, Inc. (790523323) Establishment Name Address ID/FEI Business Operations AptaPharma, Inc. 790523323 manufacture(76281-507)