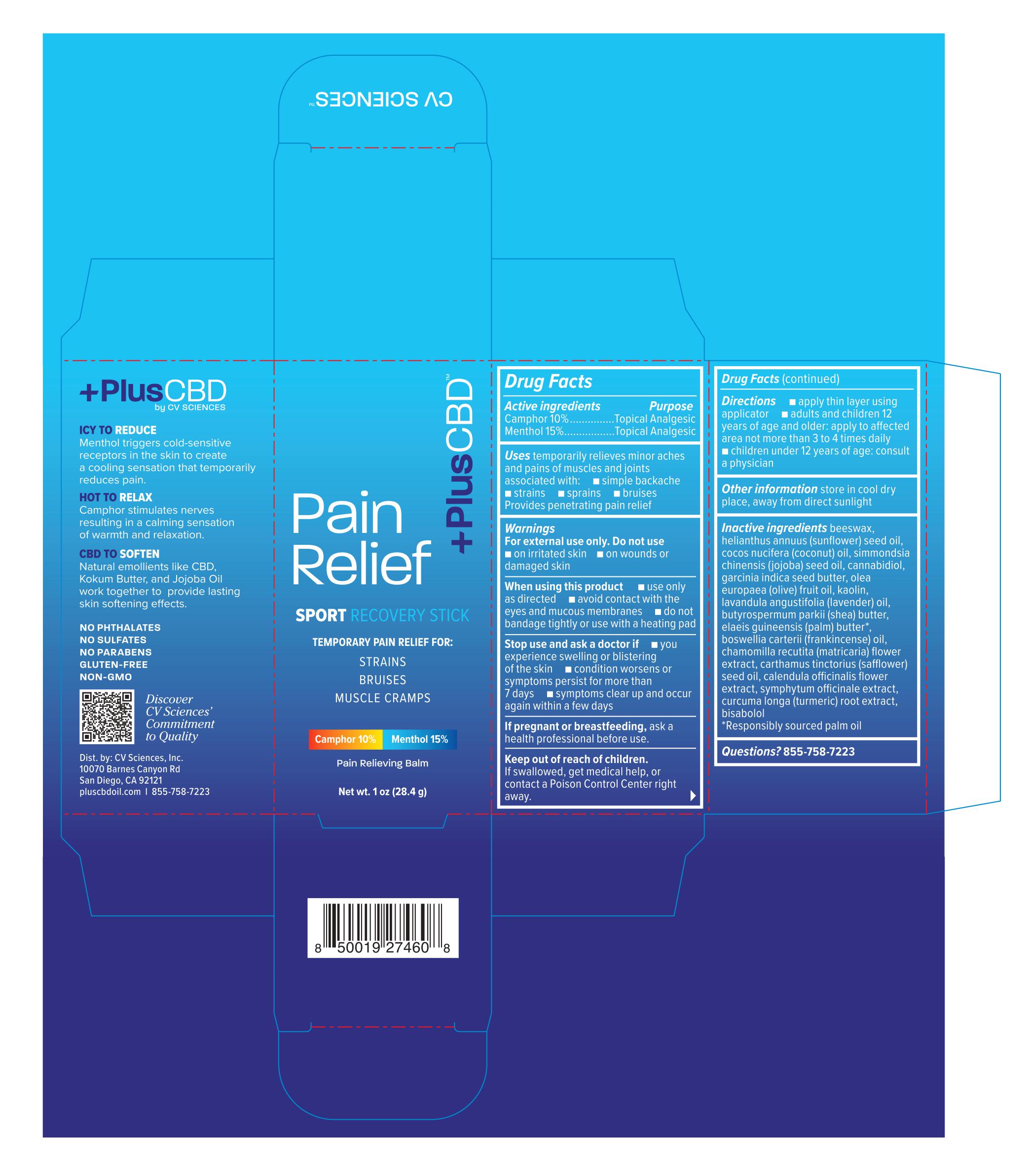
Label: PLUSCBD SPORT RECOVERY STICK 1OZ- camphor, menthol cream
- NDC Code(s): 82026-612-56
- Packager: CV Sciences, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 20, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only.
When using this product
- use only as directed
- avoid contact with the eyes and mucous membranes
- do not bandage tightly or use with a heating pad
- Directions
- Other information
-
Inactive ingredients
beeswax, helianthus annuus (sunflower) seed oil, cocos nucifera (coconut) oil, simmondsia chinensis (jojoba) seed oil, cannabidiol, garcinia indica seed butter, olea europaea (olive) fruit oil, kaolin, lavandula angustifolia (lavender) oil, butyrospermum parkii (shea) butter, elaeis guineensis (palm) butter*, boswellia carterii (frankincense) oil, chamomilla recutita (matricaria) flower extract, carthamus tinctorius (safflower) seed oil, calendula officinalis flower extract, symphytum officinale extract, curcuma longa (turmeric) root extract, bisbolol
*Responsibly sourced palm oil
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PLUSCBD SPORT RECOVERY STICK 1OZ
camphor, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82026-612 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 10 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 15 g in 100 g Inactive Ingredients Ingredient Name Strength COCONUT OIL (UNII: Q9L0O73W7L) SHEA BUTTER (UNII: K49155WL9Y) CHAMOMILE (UNII: FGL3685T2X) TURMERIC (UNII: 856YO1Z64F) SYMPHYTUM OFFICINALE WHOLE (UNII: H8FJJ6KX5Y) YELLOW WAX (UNII: 2ZA36H0S2V) JOJOBA OIL (UNII: 724GKU717M) OLIVE OIL (UNII: 6UYK2W1W1E) LAVENDER OIL (UNII: ZBP1YXW0H8) SUNFLOWER OIL (UNII: 3W1JG795YI) CANNABIDIOL (UNII: 19GBJ60SN5) GARCINIA INDICA SEED BUTTER (UNII: US2H3D7800) FRANKINCENSE OIL (UNII: 67ZYA5T02K) SAFFLOWER SEED OIL POLYGLYCERYL-6 ESTERS (UNII: R2Z4507WJN) KAOLIN (UNII: 24H4NWX5CO) ELAEIS GUINEENSIS FRUIT BUTTER (UNII: UYH3R74N56) LEVOMENOL (UNII: 24WE03BX2T) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82026-612-56 1 in 1 CARTON 08/23/2021 1 28.4 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/23/2021 Labeler - CV Sciences, Inc. (060408113) Establishment Name Address ID/FEI Business Operations Filltech USA, LLC 926433855 manufacture(82026-612)