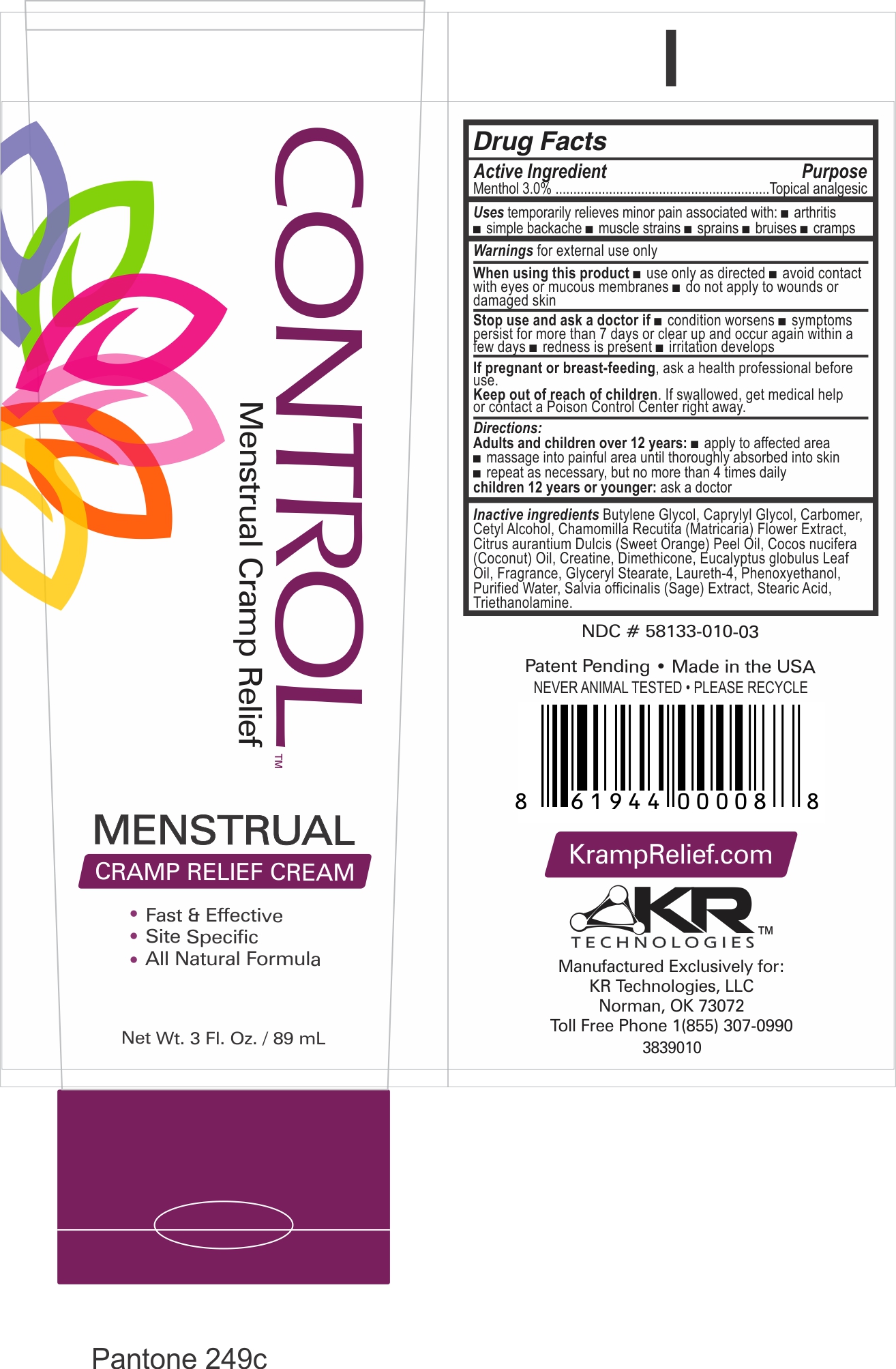
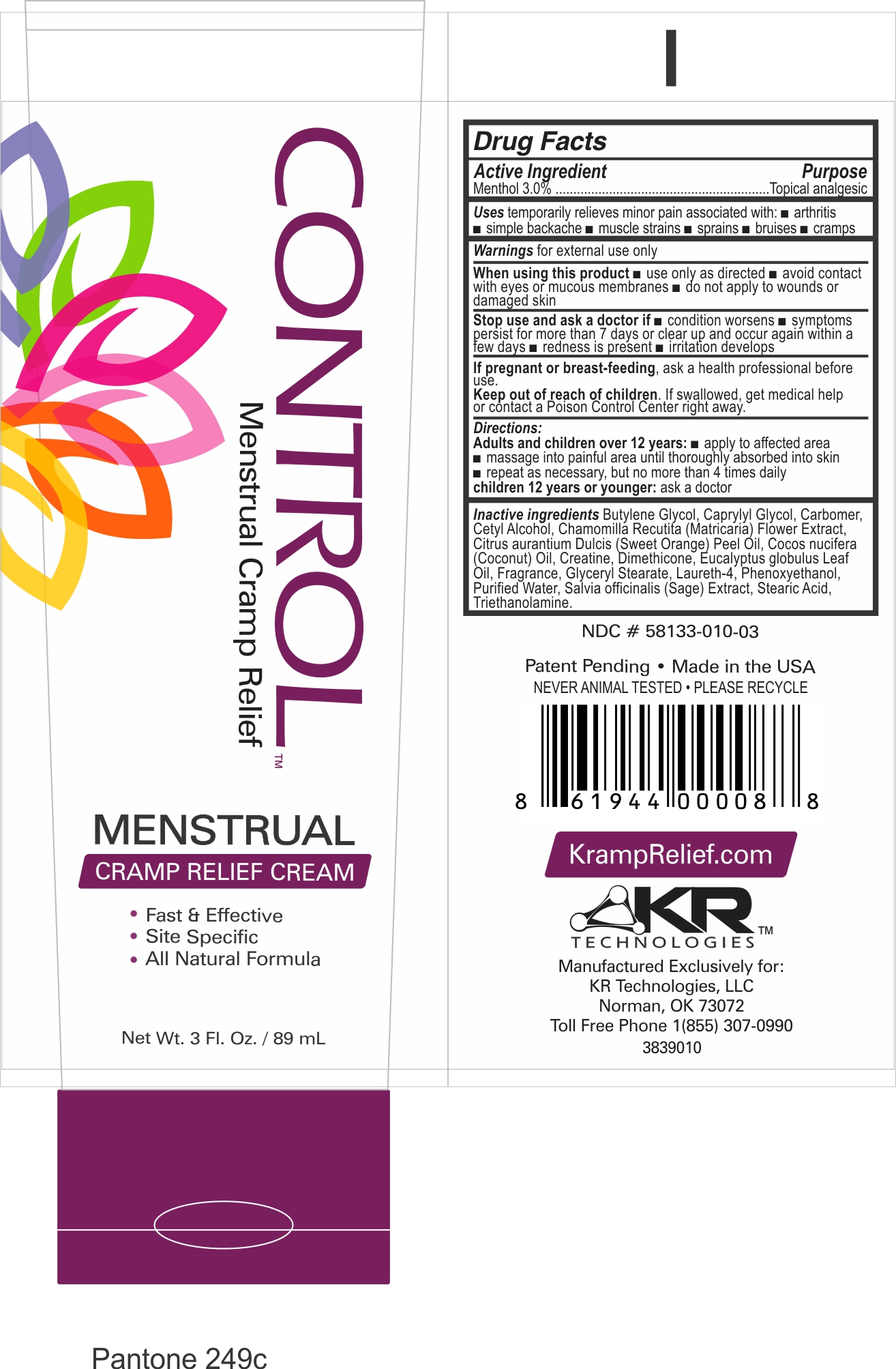
Label: CONTROL MENSTRUAL CRAMP RELIEF- menthol cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 58133-010-03 - Packager: Cosmetic Specialty Labs, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 19, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Incredient
- Purpose
- Uses
- Warnings
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions:
-
Inactive ingredients
Butylene Glycol, Caprylyl Glycol, Carbomer, Cetyl Alcohol, Chamomilla recutita (Matricaria) Flower Extract, Citrus aurantium Dulcis (Sweet Orange) Peel Oil, Cocos nucifera (Coconut) Oil, Creatine, Dimethicone, Eucalyptus globulus Leaf Oil, Fragrance, Glyceryl Stearate, Laureth-4, Phenoxyethanol, Purified Water, Salvia officinalis (Sage) Extract, Stearic Acid, Triethanolamine.
- Package Label
-
INGREDIENTS AND APPEARANCE
CONTROL MENSTRUAL CRAMP RELIEF
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58133-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETYL ALCOHOL (UNII: 936JST6JCN) CHAMOMILE (UNII: FGL3685T2X) ORANGE OIL, COLD PRESSED (UNII: AKN3KSD11B) COCONUT OIL (UNII: Q9L0O73W7L) LAURETH-4 (UNII: 6HQ855798J) SAGE (UNII: 065C5D077J) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) CREATINE (UNII: MU72812GK0) DIMETHICONE 200 (UNII: RGS4T2AS00) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) WATER (UNII: 059QF0KO0R) CARBOMER 940 (UNII: 4Q93RCW27E) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58133-010-03 89 mL in 1 TUBE; Type 0: Not a Combination Product 03/04/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/04/2021 Labeler - Cosmetic Specialty Labs, Inc. (032973000) Establishment Name Address ID/FEI Business Operations Cosmetic Specialty Labs, Inc. 032973000 manufacture(58133-010) , pack(58133-010) , label(58133-010) , analysis(58133-010)