Label: CETIRIZINE HYDROCHLORIDE (ALLERGY)- cetirizine hydrochloride tablet
- NDC Code(s): 16714-799-01, 16714-799-02, 16714-799-03, 16714-799-04
- Packager: NorthStar Rx LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 29, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
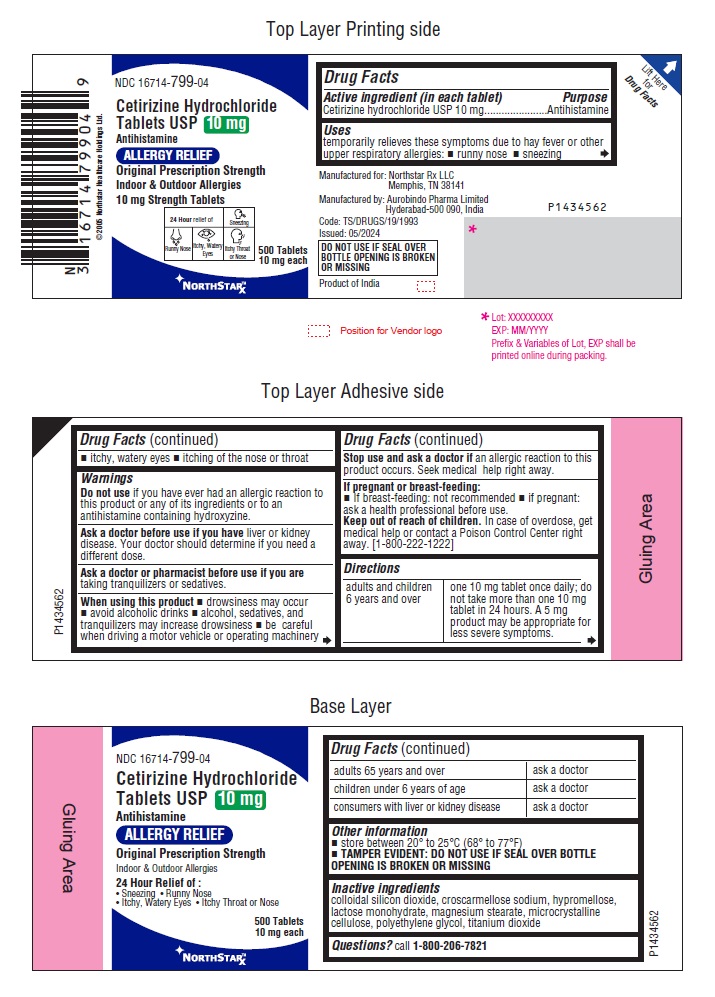
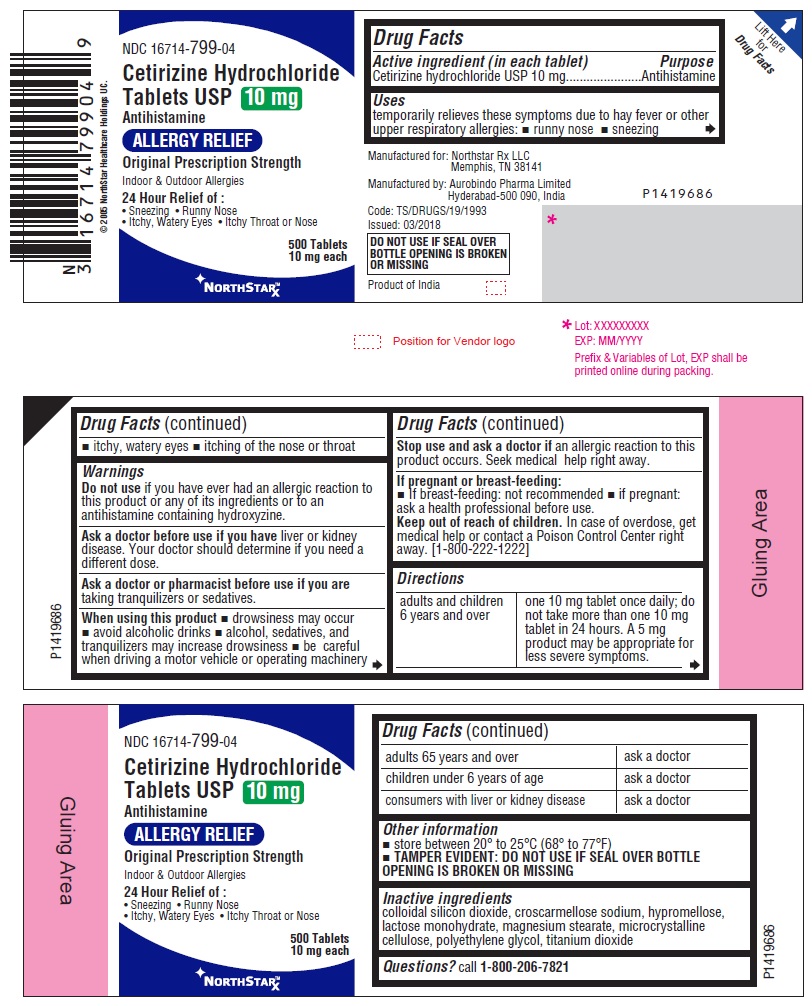
- Active ingredient (in each tablet)
- Purpose
- Uses
- Warnings
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding:
- Keep out of reach of children.
-
Directions
adults and children
6 years and over
one 10 mg tablet once daily;
do not take more than one 10 mg
tablet in 24 hours. A 5 mg
product may be appropriate for
less severe symptoms.
adults 65 years and over
ask a doctor
children under 6 years of age
ask a doctor
consumers with liver or
kidney disease
ask a doctor
- Other information
- Inactive ingredients
- Questions?
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg (500's Tablets Container Label)
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE (ALLERGY)
cetirizine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:16714-799 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (White to Off-white) Score no score Shape ROUND Size 8mm Flavor Imprint Code X;36 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16714-799-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/05/2015 2 NDC:16714-799-02 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/05/2015 3 NDC:16714-799-03 300 in 1 BOTTLE; Type 0: Not a Combination Product 08/05/2015 4 NDC:16714-799-04 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/05/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090760 08/05/2015 Labeler - NorthStar Rx LLC (830546433) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 918917642 ANALYSIS(16714-799) , MANUFACTURE(16714-799)