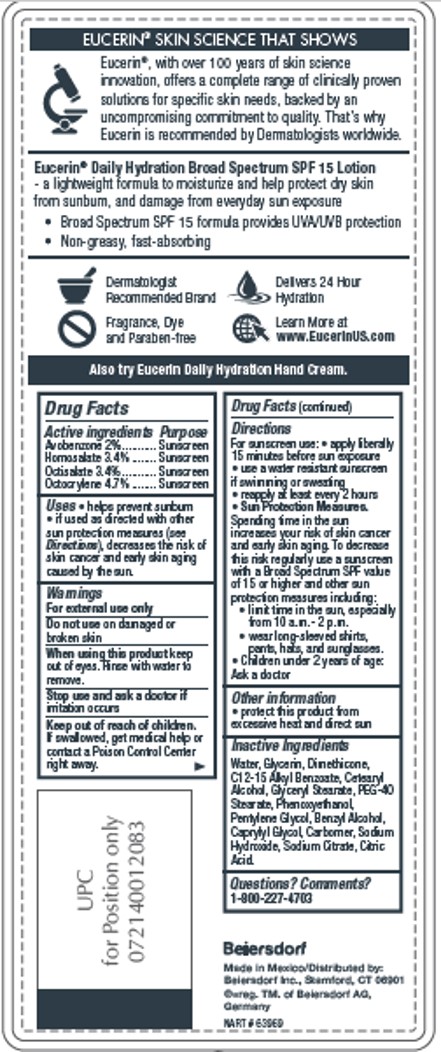
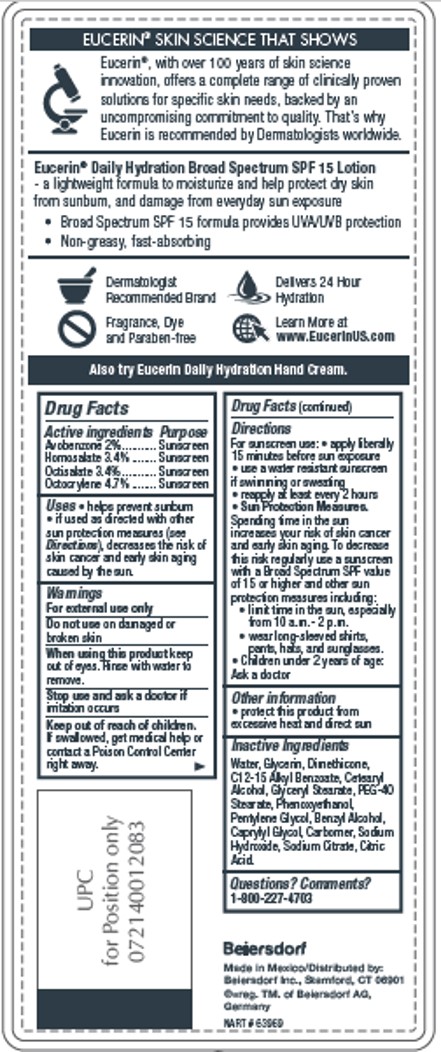
Label: EUCERIN DAILY HYDRATION- avobenzone, homosalate, octisalate, octocrylene lotion
- NDC Code(s): 10356-328-31
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
For sunscreen use: • apply liberally 15
minutes before sun exposure • use a water
resistant sunscreen if swimming or sweating
• reapply at least every 2 hours
• Sun Protection Measures. Spending time in
the sun increases your risk of skin cancer and
early skin aging. To decrease this risk regularly
use a sunscreen with a Broad Spectrum SPF
value of 15 or higher and other sun protection
measures including:
• limit time in the sun, especially from
10 a.m.-2 p.m.• wear long-sleeved shirts,
pants, hats, and sunglasses.
• Children under 6 months of age: Ask a doctor - INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EUCERIN DAILY HYDRATION
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10356-328 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 3.4 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 3.4 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4.7 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIMETHICONE (UNII: 92RU3N3Y1O) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-40 STEARATE (UNII: ECU18C66Q7) PENTYLENE GLYCOL (UNII: 50C1307PZG) BENZYL ALCOHOL (UNII: LKG8494WBH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM CITRATE (UNII: 1Q73Q2JULR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CARBOMER HOMOPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: F68VH75CJC) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10356-328-31 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/01/2016 Labeler - Beiersdorf Inc (001177906)