Label: LILIKOI LIGHT DEFENSE FACE PRIMER SPF 23- zinc oxide, titanium dioxide lotion
- NDC Code(s): 15751-2323-2, 15751-2323-9
- Packager: Eminence Organic Skin Care Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
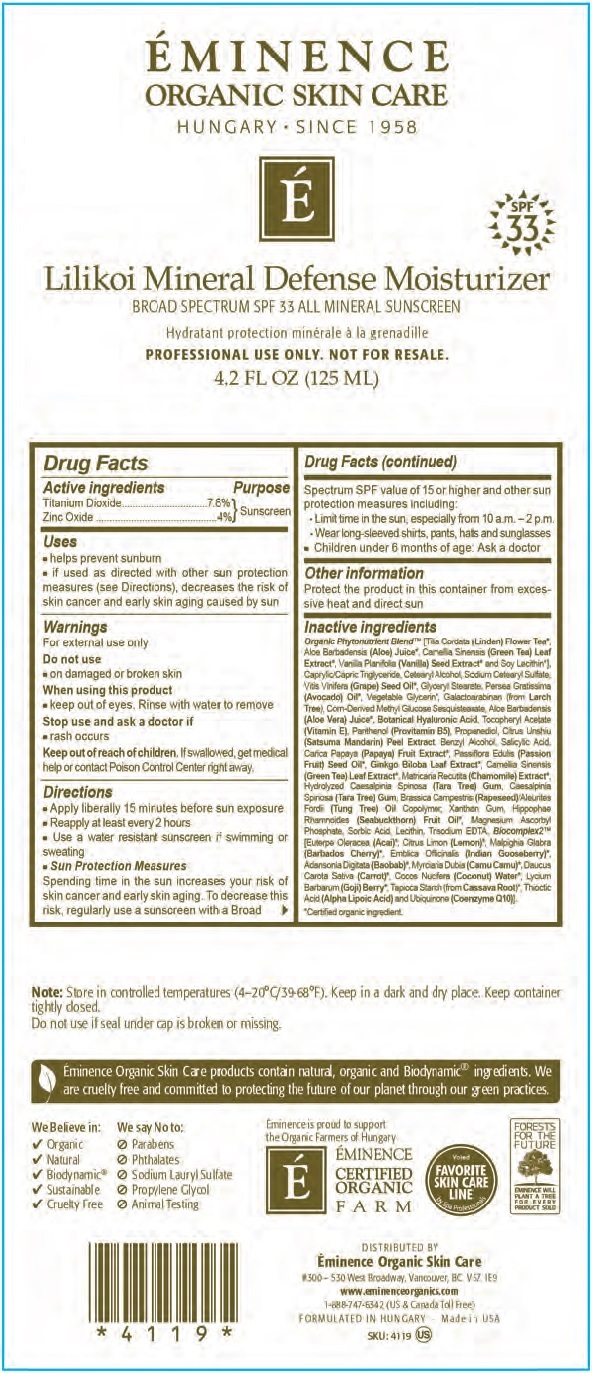
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
- Apply liberally and evenly to cleansed skin or after your moisturizer, 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water-resistant sunscreen if swimming or sweating
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses
- Children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
Organic Phytonutrient Blend [(Tilia Cordata (Linden) Flower Tea, Aloe Barbadensis (Aloe) Juice, Camellia Sinensis (Green Tea) Leaf Extract, Vanilla Planifolia (Vanilla) Seed Extract and Soy Lecithin, Caprylic/Capric Triglyceride, Cetearyl Alcohol, Sodium Cetearyl Sulfate, Vitis Vinifera (Grape) Seed Oil, Glyceryl Stearate, Persea Gratissima (Avocado) Oil, Vegetable Glycerin, Silica, Cellulose, Mica, Barium Sulfate, Titanium Dioxide, Simmondsia Chinensis (Jojoba) Esters, Galactoarabinan (from Larch Tree), Aloe Barbadensis (Aloe Vera) Juice, Botanical Hyaluronic Acid, Tocopheryl Acetate (Vitamin E), Panthenol (Provitamin B5), Theobroma Cacao (Cocoa) Seed Extract, Benzyl Alcohol, Salicylic Acid, Carica Papaya (Papaya) Fruit Extract, Ginkgo Biloba Leaf Extract, Camellia Sinensis (Green Tea) Leaf Extract, Matricaria Recutita (Chamomile) Extract, Passiflora Edulis (Passion Fruit) Seed Oil, Polyacrylate Crosspolymer-6, Xanthan Gum, Hippophae Rhamnoides (Seabuckthorn) Fruit Oil, Corn-Derived Methyl Glucose Sesquistearate, Magnesium Ascorbyl Phosphate, Sorbic Acid, Lecithin, Polyhydroxystearic Acid, Isostearic Acid, Polyglyceryl-3 Polyricinoleate, Trisodium EDTA, Biocomplex2 (Euterpe Oleracea (Acai), Citrus Limon (Lemon), Malpighia Glabra (Barbados Cherry), Emblica Officinalis (Indian Gooseberry), Adansonia Digitata (Baobab), Myrciaria Dubia (Camu Camu), Daucus Carota Sativa (Carrot), Cocos Nucifera (Coconut), Water, Lycium Barbarum (Goji) Berry, Tapioca Starch From Cassava Root, Thioctic Acid (Alpha Lipoic Acid) and Ubiquinone (Coenzyme Q10)]. Cerified organic ingredient.
- Note
- Lilikoi Light Defense Face Primer SPF 23, 1.2 fl. oz (15751-2323-2)
- Lilikoi Light Defense Face Primer SPF 23, 1.9 fl. oz 15751-2323-9
-
INGREDIENTS AND APPEARANCE
LILIKOI LIGHT DEFENSE FACE PRIMER SPF 23
zinc oxide, titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:15751-2323 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 70 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE (UNII: V5VD430YW9) GREEN TEA LEAF (UNII: W2ZU1RY8B0) VANILLA (UNII: Q74T35078H) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SODIUM CETOSTEARYL SULFATE (UNII: 7ZBS06BH4B) GRAPE SEED OIL (UNII: 930MLC8XGG) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) AVOCADO OIL (UNII: 6VNO72PFC1) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICA (UNII: V8A1AW0880) BARIUM SULFATE (UNII: 25BB7EKE2E) GALACTOARABINAN (UNII: SL4SX1O487) ALOE VERA LEAF (UNII: ZY81Z83H0X) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) PANTHENOL (UNII: WV9CM0O67Z) COCOA (UNII: D9108TZ9KG) BENZYL ALCOHOL (UNII: LKG8494WBH) SALICYLIC ACID (UNII: O414PZ4LPZ) PAPAYA (UNII: KU94FIY6JB) GINKGO (UNII: 19FUJ2C58T) CHAMOMILE (UNII: FGL3685T2X) PASSIFLORA EDULIS SEED OIL (UNII: F3VOA31UHQ) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) XANTHAN GUM (UNII: TTV12P4NEE) HIPPOPHAE RHAMNOIDES FRUIT OIL (UNII: TA4JCF9S1J) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) SORBIC ACID (UNII: X045WJ989B) ISOSTEARIC ACID (UNII: X33R8U0062) EDETATE TRISODIUM (UNII: 420IP921MB) ACAI (UNII: 46AM2VJ0AW) LEMON (UNII: 24RS0A988O) CARROT (UNII: L56Z1JK48B) COCONUT (UNII: 3RT3536DHY) WATER (UNII: 059QF0KO0R) THIOCTIC ACID (UNII: 73Y7P0K73Y) UBIDECARENONE (UNII: EJ27X76M46) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15751-2323-2 1 in 1 BOX 03/01/2019 1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:15751-2323-9 1 in 1 BOX 03/01/2019 2 55 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/01/2019 Labeler - Eminence Organic Skin Care Ltd. (205753317)