Label: 3M DURAPREP SURGICAL- iodine povacrylex and isopropyl alcohol solution
- NDC Code(s): 17518-011-08
- Packager: Solventum US OpCo LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only. Flammable, keep away from fire or flame.
To reduce the risk of fire, PREP CAREFULLY:
- do not use 26-mL applicator for head and neck surgery
- do not use on an area smaller than 8 in. x 10 in. Use a small applicator instead.
- solution contains alcohol and gives off flammable vapors
- do not drape or use ignition source (e.g., cautery, laser) until solution is completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair).
- avoid getting solution into hairy areas. Wet hair is flammable. Hair may take up to 1 hour to dry.
- do not allow solution to pool
- remove solution-stained material from prep area
Do not use
- on patients with known allergies to iodine or any other ingredients in this product
- on open wounds, on mucous membranes, or as a general skin cleanser
- in infants less than 2 months old due to the risk of excessive skin irritation and transient hypothyroidism
When using this product
- keep out of eyes, ears, and mouth. May cause serious injury if permitted to enter and remain. If contact occurs, flush with cold water right away and contact a doctor.
- to avoid skin injury, care should be taken when removing drapes, tapes, etc…applied over film
- use with caution in women who are breast-feeding due to the potential for transient hypothyroidism in the nursing newborn
-
Directions (follow all directions for use)
- at the end of the prep, discard any portion of the solution which is not required to cover the prep area. It is not necessary to use the entire amount available.
Getting Patient Ready for Solution:
- use in well-ventilated area
- do not microwave or heat the solution applicator
- apply to clean, completely dry, residue-free, intact skin
- when hair removal is necessary, use a surgical clipper on the morning of the surgery. If a wet shave is used, thoroughly remove all soap residues.
Activating the Applicator:
- with sponge face parallel to the floor, press the cap end of the applicator. Solution will begin to flow into the sponge.
- wait for fluid level to reach indicator line of applicator barrel
When Applying Solution:
- DO NOT SCRUB. Paint a single, uniform application and do not reprep area.
- do not allow solution to pool. Use sponge applicator to absorb excess solution and continue to apply a uniform coating. If solution accidentally gets outside of prep area, remove excess with gauze.
- clean umbilicus with enclosed swabs when applicable. (Moisten swabs by pressing against solution-soaked sponge applicator.)
- tuck prep towels as needed under both sides of the neck to absorb excess solution. Remove towels before draping.
- avoid getting solution into hairy areas. Wet hair is flammable. Hair may take up to 1 hour to dry.
- when prepping skin folds, toes, or fingers, use a sterile-gloved hand to hold skin apart until completely dry. Otherwise, skin may adhere to itself.
After Applying Solution:
- to reduce the risk of fire, wait until solution is completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair). Solution will turn from a shiny to a dull appearance on skin alerting the user that the solution is completely dry and no longer flammable.
While Waiting for Solution to Completely Dry:
- do not drape or use ignition source (e.g., cautery, laser)
- check for pooled solution. Use sterile gauze to soak up pooled solution.
- Do not blot because it may remove solution from skin.
- remove solution-stained materials. Replace if necessary.
After Solution is Completely Dry:
- to reduce the risk of fire, begin draping and/or using cautery only after solution is completely dry and all solution-stained materials are removed
- if incise drapes are used, apply directly to dry prep. On completion of surgical procedure, removal of incise drape will remove film.
- apply dressing following standard practices
- Other information
- Inactive ingredients
- Questions?
-
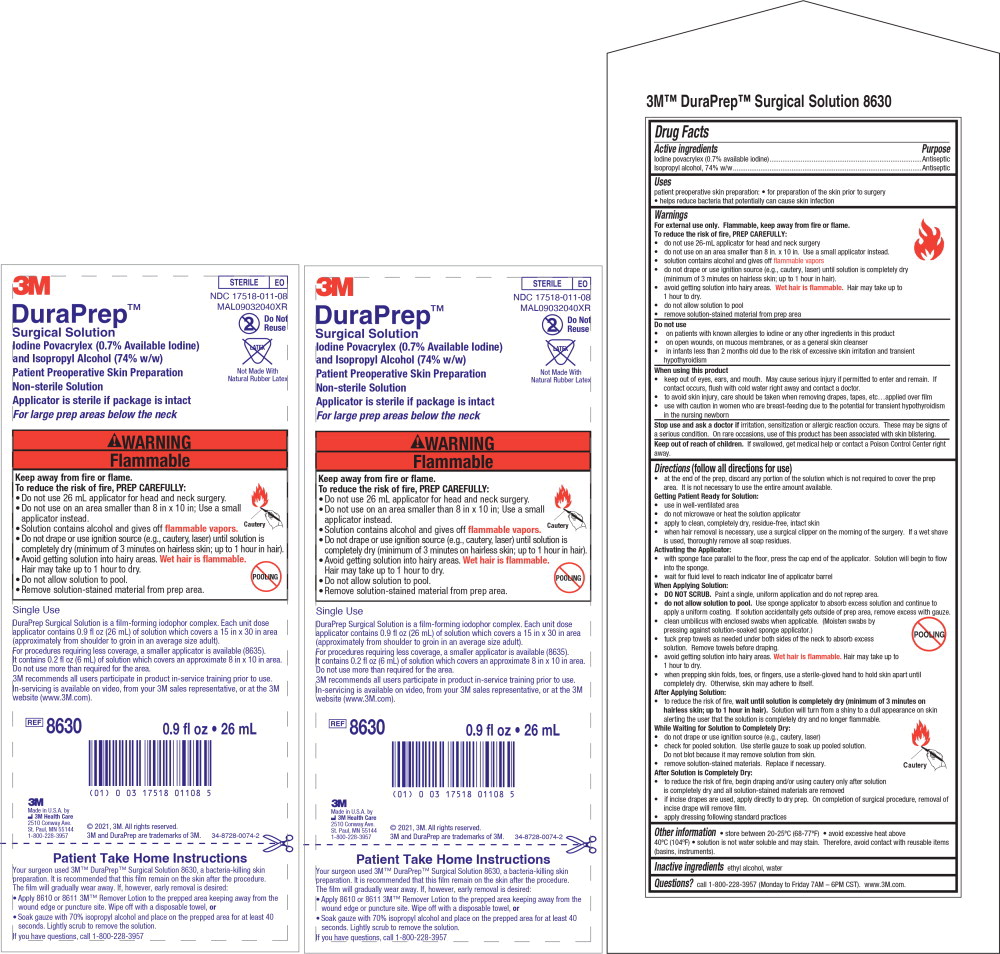
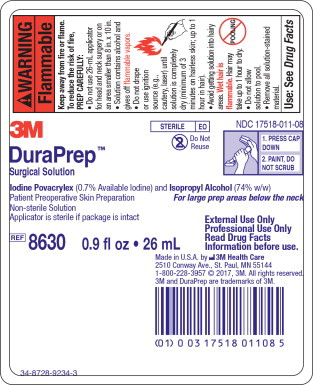
Principle Display Panel – 26 mL Applicator Label
3M
STERILE EO
NDC 17518-011-08
Do Not Reuse
1. PRESS CAP
DOWN
2. PAINT, DO
NOT SCRUB
DuraPrepTM
Surgical Solution
Iodine Povacrylex (0.7% Available Iodine) and Isopropyl Alcohol (74% w/w)
Patient Preoperative Skin Preparation For large prep areas below the neck
Non-Sterile Solution
Applicator is sterile if package is intact
REF 8630 0.9 fl oz • 26 mL
External Use Only
Professional Use Only
Read Drug Facts Information before use.
Made in U.S.A. by 3M Health Care
2510 Conway Ave., St. Paul, MN 55144
1-800-228-3957 © 2017, 3M. All rights reserved.
3M and DuraPrep are trademarks of 3M.
-
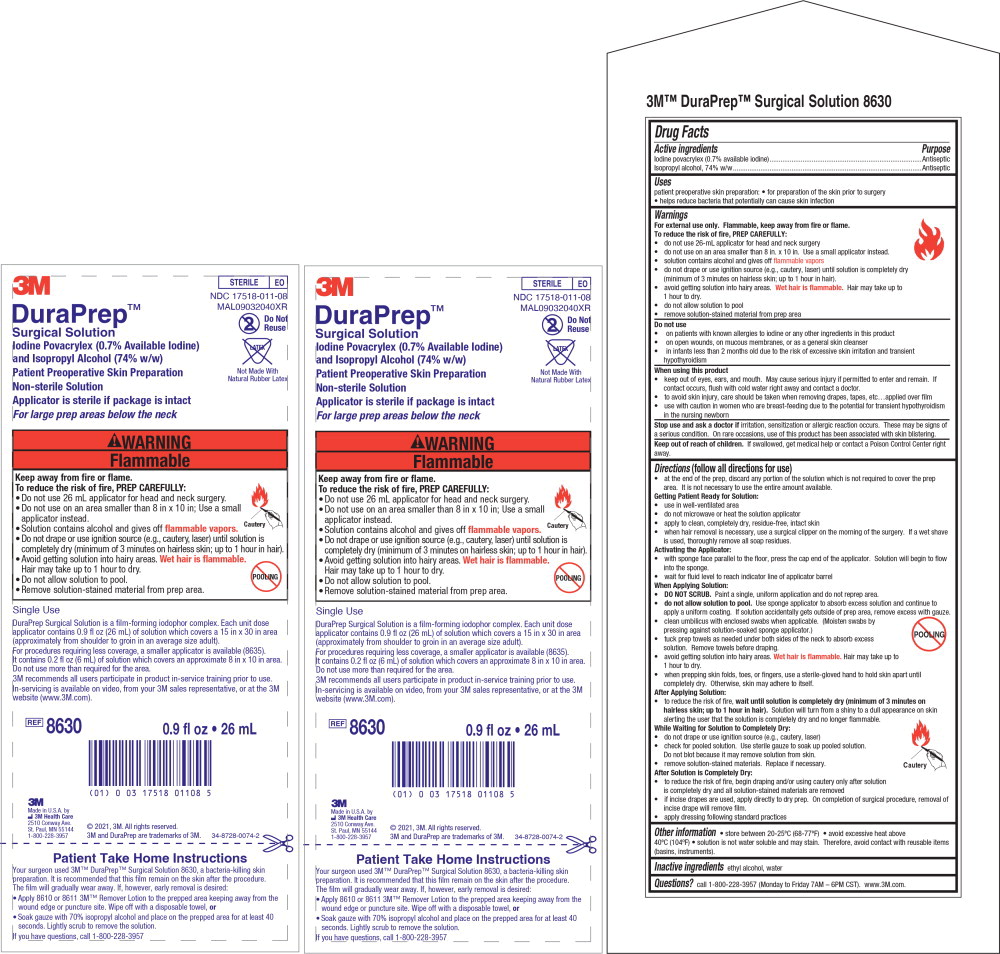
Principal Display Panel – 26 mL Insert Label
3M
STERILE EO
NDC 17518-011-08
MAL09032040XR
DuraPrep TM
Surgical Solution
Iodine Povacrylex (0.7% Available Iodine)
and Isopropyl Alcohol (74% w/w)
Patient Preoperative Skin Preparation
Non-sterile Solution
Applicator is sterile if package is intact
For large prep areas below the neck
Do Not Reuse
Latex-Free
Not Made With Natural Rubber Latex
Single Use
DuraPrep Surgical Solution is a film-forming iodophor complex. Each unit dose
applicator contains 0.9 fl oz (26 mL) of solution which covers a 15 in x 30 in area
(approximately from shoulder to groin in an average size adult).
For procedures requiring less coverage, a smaller applicator is available (8635). It
contains 0.2 fl oz (6 mL) of solution which covers an approximate 8 in x 10 in area.
Do not use more than required for the area.
3M recommends all users participate in product in-service training prior to use.
In-servicing is available on video, from your 3M sales representative, or at the 3M
website (www.3M.com).
REF 8630 0.9 fl oz • 26 mL
3M
Made in U.S.A. by
3M Health Care
2510 Conway Ave.
St. Paul, MN 55144
1-800-228-3957
© 2021, 3M. All rights reserved.
3M and DuraPrep are trademarks of 3M.
-
INGREDIENTS AND APPEARANCE
3M DURAPREP SURGICAL
iodine povacrylex and isopropyl alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17518-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Iodine Povacrylex (UNII: 6E43AWY083) (Iodine - UNII:9679TC07X4) Iodine 6.02 mg in 1 mL Isopropyl Alcohol (UNII: ND2M416302) (Isopropyl Alcohol - UNII:ND2M416302) Isopropyl Alcohol 636.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17518-011-08 1 in 1 CASE 09/29/2006 1 26 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021586 09/29/2006 Labeler - Solventum US OpCo LLC (006173082) Establishment Name Address ID/FEI Business Operations 3M Company 054950670 ANALYSIS(17518-011) , PACK(17518-011) Establishment Name Address ID/FEI Business Operations 3M Company 078671244 MANUFACTURE(17518-011) Establishment Name Address ID/FEI Business Operations 3M Company 830016148 ANALYSIS(17518-011)