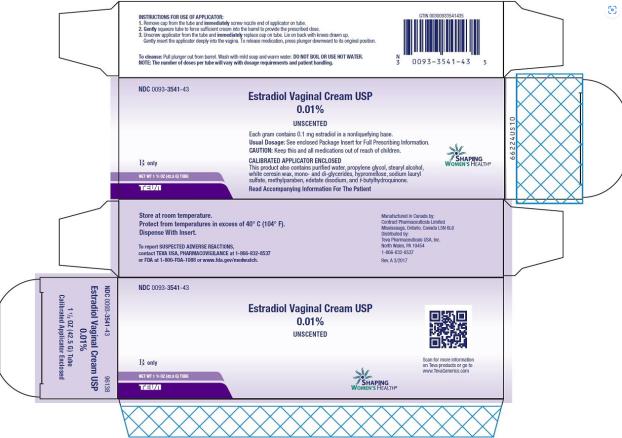
Label: ESTRADIOL cream
- NDC Code(s): 0093-3541-43
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, BREAST CANCER and PROBABLE DEMENTIA
Estrogen-Alone Therapy
Endometrial Cancer
There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding [see WARNINGS, Malignant Neoplasms, Endometrial Cancer].
Cardiovascular Disorders and Probable Dementia
Estrogen-alone therapy should not be used for the prevention of cardiovascular disease or dementia [see CLINICAL STUDIES and WARNINGS, Cardiovascular Disorders, and Probable Dementia].
The Women’s Health Initiative (WHI) estrogen-alone substudy reported increased risks of stroke and deep vein thrombosis (DVT) in postmenopausal women (50 to 79 years of age) during 7.1 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg]-alone, relative to placebo [see CLINICAL STUDIES and WARNINGS, Cardiovascular Disorders].
The WHI Memory Study (WHIMS) estrogen-alone ancillary study of WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 5.2 years of treatment with daily CE (0.625 mg) -alone, relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see CLINICAL STUDIES and WARNINGS, Probable Dementia and PRECAUTIONS, Geriatric Use].
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and other dosage forms of estrogens.
Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Estrogen Plus Progestin Therapy
Cardiovascular Disorders and Probable Dementia
Estrogen plus progestin therapy should not be used for the prevention of cardiovascular disease or dementia [see CLINICAL STUDIES and WARNINGS, Cardiovascular Disorders, and Probable Dementia].
The WHI estrogen plus progestin substudy reported increased risks of DVT, pulmonary embolism (PE), stroke and myocardial infarction (MI) in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral CE (0.625 mg) combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo [see CLINICAL STUDIES and WARNINGS, Cardiovascular Disorders].
The WHIMS estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see CLINICAL STUDIES and WARNINGS, Probable Dementia and PRECAUTIONS, Geriatric Use].
Breast Cancer
The WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer [see CLINICAL STUDIES and WARNINGS, Malignant Neoplasms, Breast Cancer].
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and MPA, and other combinations and dosage forms of estrogens and progestins.
Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
-
DESCRIPTION
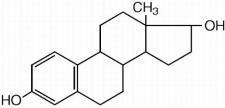
Each gram of estradiol vaginal cream USP 0.01% contains 0.1 mg estradiol in a nonliquefying base containing purified water, propylene glycol, stearyl alcohol, white ceresin wax, mono- and di-glycerides, hypromellose 2208 (4000 cps), sodium lauryl sulfate, methylparaben, edetate di-sodium and tertiary-butylhydroquinone. Estradiol is chemically described as estra-1,3,5(10)-triene-3, 17(beta)-diol. It has an empirical formula of C18H24O2 and molecular weight of 272.37. The structural formula is:
-
CLINICAL PHARMACOLOGY
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone by peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, two estrogen receptors have been identified. These vary in proportion from tissue to tissue.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.
Pharmacokinetics
Absorption
Estrogen drug products are absorbed through the skin, mucous membranes, and the gastrointestinal tract after release from the drug formulation.
Distribution
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estrogens circulate in the blood largely bound to sex hormone binding globulin (SHBG) and albumin.
Metabolism
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is the major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the gut followed by reabsorption. In postmenopausal women, a significant proportion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
Excretion
Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates.
Special Populations
No pharmacokinetic studies were conducted in special populations, including patients with renal or hepatic impairment.
Drug Interactions
In vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism. Inducers of CYP3A4 such as St. John’s Wort preparations (Hypericum perforatum), phenobarbital, carbamazepine, and rifampin may reduce plasma concentrations of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. Inhibitors of CYP3A4 such as erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir and grapefruit juice may increase plasma concentrations of estrogens and may result in side effects.
-
CLINICAL STUDIES
Women’s Health Initiative Studies
The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) (defined as nonfatal MI, silent MI and CHD death), with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other cause. These substudies did not evaluate the effects of CE or CE plus MPA on menopausal symptoms.
WHI Estrogen-Alone Substudy
The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints.
Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79; 75.3 percent White, 15.1 percent Black, 6.1 percent Hispanic, 3.6 percent Other), after an average follow-up of 7.1 years are presented in Table 1.
TABLE 1 -Relative and Absolute Risk Seen in the Estrogen-Alone Substudy of WHIa
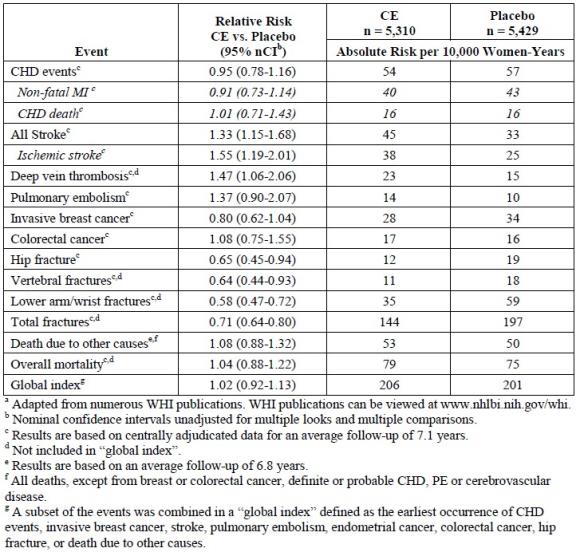
For those outcomes included in the WHI "global index" that reached statistical significance, the absolute excess risk per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures1. The absolute excess risk of events included in the "global index" was a non-significant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years.
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant differences in distribution of stroke subtypes or severity, including fatal strokes, in women receiving CE-alone compared to placebo. Estrogen-alone increased the risk for ischemic stroke, and this excess risk was present in all subgroups of women examined2.
Timing of the initiation of estrogen-alone therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age a non-significant trend toward reduced risk for CHD [hazard ratio (HR) 0.63 (95 percent CI, 0.36-1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46– 1.11)].
WHI Estrogen Plus Progestin Substudy
The WHI estrogen plus progestin substudy was also stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of breast cancer and cardiovascular events exceeded the specified benefits included in the “global index.” The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women-years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reductions per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the CE plus MPA substudy, which included 16,608 women (average 63 years of age, range 50 to 79; 83.9 percent White, 6.8 percent Black, 5.4 percent Hispanic, 3.9 percent Other), are presented in Table 2. These results reflect centrally adjudicated data after an average follow- up of 5.6 years.
TABLE 2 -Relative and Absolute Risk Seen in the Estrogen Plus Progestin Substudy of WHI at an Average of 5.6 Yearsa,b
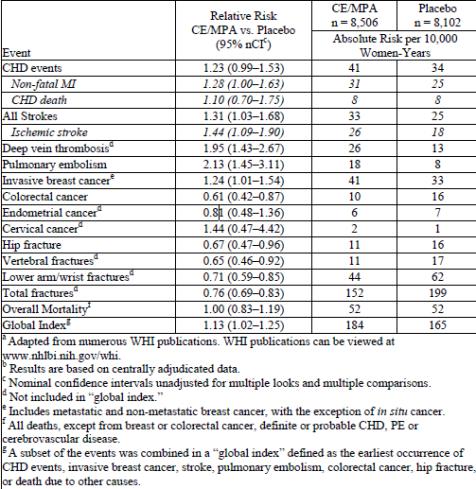
Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age, a non-significant trend toward reduced risk for overall mortality [HR 0.69 (95 percent CI, 0.44-1.07)].
Women’s Health Initiative Memory Study
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age and older (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83-2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in this study included Alzheimer’s disease (AD), vascular dementia (VaD) and mixed types (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women [see BOXED WARNINGS, WARNINGS, Probable Dementia and PRECAUTIONS, Geriatric Use].
The WHIMS estrogen plus progestin ancillary study of WHI enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years; 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21-3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 per 10,000 women-years. Probable dementia as defined in this study included AD, VaD and mixed types (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women [see WARNINGS, Probable Dementia and PRECAUTIONS, Geriatric Use].
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19-2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women [see WARNINGS, Probable Dementia and PRECAUTIONS, Geriatric Use].
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Estradiol vaginal cream USP 0.01% should not be used in women with any of the following conditions:
- Undiagnosed abnormal genital bleeding.
- Known, suspected, or history of cancer of the breast.
- Known or suspected estrogen-dependent neoplasia.
- Active DVT, PE or history of these conditions.
- Active arterial thromboembolic disease (for example, stroke, MI) or a history of these conditions.
- Known anaphylactic reaction or angioedema to estradiol vaginal cream USP 0.01%.
- Known liver dysfunction or disease.
- Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders.
- Known or suspected pregnancy.
- Undiagnosed abnormal genital bleeding.
-
WARNINGS
See BOXED WARNINGS.
Systemic absorption may occur with the use of estradiol vaginal cream USP 0.01%. The warnings, precautions, and adverse reactions associated with oral estrogen treatment should be taken into account.
1. Cardiovascular Disorders
An increased risk of stroke and DVT has been reported with estrogen-alone therapy. An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (e.g., hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (e.g., personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
a. Stroke
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). The increase in risk was demonstrated in year one and persisted [see CLINICAL STUDIES]. Should a stroke occur or be suspected, estrogen-alone therapy should be discontinued immediately.
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years)3.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women- years) [see CLINICAL STUDIES]. The increase in risk was demonstrated after the first year and persisted3. Should a stroke occur or be suspected, estrogen plus progestin therapy should be discontinued immediately.
b. Coronary Heart Disease
In the WHI estrogen-alone substudy, no overall effect on coronary heart disease (CHD) events (defined as nonfatal MI, silent MI and CHD death) was reported in women receiving estrogen-alone compared to placebo4 [see CLINICAL STUDIES]. Subgroup analyses of women 50 to 59 years of age suggest a statistically non-significant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women with less than 10 years since menopause (8 versus 16 per 10,000 women-years) 3.
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women-years) 3. An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 [see CLINICAL STUDIES].
In postmenopausal women with documented heart disease (n = 2,763), average age 66.7 years of age, in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established coronary heart disease. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years.
Two thousand, three hundred and twenty one (2,321) women from the original HERS trial agreed to participate in an open label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in HERS, HERS II, and overall.
c. Venous Thromboembolism
In the WHI estrogen-alone substudy, the risk of VTE (DVT and PE) was increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women-years). The increase in VTE risk was demonstrated during the first 2 years5 [see CLINICAL STUDIES]. Should a VTE occur or be suspected, estrogen-alone therapy should be discontinued immediately.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women-years) were also demonstrated. The increase in VTE risk was observed during the first year and persisted6 [see CLINICAL STUDIES]. Should a VTE occur or be suspected, estrogen plus progestin therapy should be discontinued immediately.
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
2. Malignant Neoplasms
a. Endometrial Cancer
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among unopposed estrogen users is about 2- to 12-times greater than in non-users, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with use of estrogens for less than one year. The greatest risk appears associated with prolonged use, with increased risks of 15- to 24-fold for five to ten years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding.
There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
b. Breast Cancer
The most important randomized clinical trial providing information about breast cancer in estrogen-alone users is the WHI substudy of daily CE (0.625 mg)-alone. In the WHI estrogen-alone substudy, after an average follow-up of 7.1 years, daily CE (0.625 mg)-alone was not associated with an increased risk of invasive breast cancer (relative risk [RR] 0.80) 7 [see CLINICAL STUDIES].
The most important randomized clinical trial providing information about breast cancer in estrogen plus progestin users is the WHI substudy of daily CE (0.625 mg) plus MPA (2.5 mg). After a mean follow-up of 5.6 years, the estrogen plus progestin substudy reported an increased risk of invasive breast cancer in women who took daily CE plus MPA. In this substudy, prior use of estrogen-alone or estrogen plus progestin therapy was reported by 26 percent of the women. The relative risk of invasive breast cancer was 1.24, and the absolute risk was 41 versus 33 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported prior use of hormone therapy, the relative risk of invasive breast cancer was 1.86, and the absolute risk was 46 versus 25 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported no prior use of hormone therapy, the relative risk of invasive breast cancer was 1.09, and the absolute risk was 40 versus 36 cases per 10,000 women- years, for CE plus MPA compared with placebo. In the same substudy, invasive breast cancers were larger, were more likely to be node positive, and were diagnosed at a more advanced stage in the CE (0.625 mg) plus MPA (2.5 mg) group compared with the placebo group. Metastatic disease was rare with no apparent difference between the two groups. Other prognostic factors such as histologic subtype, grade and hormone receptor status did not differ between the groups8 [see CLINICAL STUDIES].
Consistent with the WHI clinical trial, observational studies have also reported an increased risk of breast cancer for estrogen plus progestin therapy, and a smaller increased risk for estrogen-alone therapy, after several years of use. One large meta-analysis of prospective cohort studies reported increased risks that were dependent upon duration of use and could last up to >10 years after discontinuation of estrogen plus progestin therapy and estrogen-alone therapy. Extension of the WHI trials also demonstrated increased breast cancer risk associated with estrogen plus progestin therapy. Observational studies also suggest that the risk of breast cancer was greater, and became apparent earlier, with estrogen plus progestin therapy as compared to estrogen-alone therapy. However, these studies have not generally found significant variation in the risk of breast cancer among different estrogen plus progestin combinations, doses, or routes of administration.
The use of estrogen-alone and estrogen plus progestin therapy has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
c. Ovarian Cancer
The WHI estrogen plus progestin substudy reported a statistically non-significant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI, 0.77-3.24). The absolute risk for CE plus MPA was 4 versus 3 cases per 10,000 women-years9.
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years] vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
3. Probable Dementia
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo.
After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83-2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years10 [see CLINICAL STUDIES and PRECAUTIONS, Geriatric Use].
In the WHIMS estrogen plus progestin ancillary study, a population of 4,532 postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21-3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years10 [see CLINICAL STUDIES and PRECAUTIONS, Geriatric Use].
When data from the two populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19-2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women 10 [see PRECAUTIONS, Geriatric Use].
4. Gallbladder Disease
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
5. Hypercalcemia
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
6. Visual Abnormalities
Retinal vascular thrombosis has been reported in patients receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
7. Anaphylactic Reaction and Angioedema
Cases of anaphylaxis, which develop within minutes to hours after taking orally-administered estrogen and require emergency medical management, have been reported in the postmarketing setting. Skin (hives, pruritis, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) involvement has been noted.
Angioedema involving the tongue, larynx, face, hands and feet requiring medical intervention has occurred postmarketing in patients taking orally-administered estrogen. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Patients who develop an anaphylactic reaction with or without angioedema after treatment with oral estrogen should not receive oral estrogen again.
-
PRECAUTIONS
A. General
1. Addition of a Progestin When a Woman Has Not Had a Hysterectomy
Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
There are, however, possible risks that may be associated with the use of progestins with estrogens compared to estrogen-alone regimens. These include a possible increased risk of breast cancer.
2. Elevated Blood Pressure
In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogens on blood pressure was not seen.
3. Hypertriglyceridemia
In women with pre-existing hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis and other complications. Consider discontinuation of treatment if pancreatitis occurs.
4. Hepatic Impairment and/or Past History of Cholestatic Jaundice
Estrogens may be poorly metabolized in patients with impaired liver function. For women with a history of cholestatic jaundice associated with past estrogen use or with pregnancy, caution should be exercised and in the case of recurrence, medication should be discontinued.
5. Hypothyroidism
Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Women with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T4 and T3 serum concentrations in the normal range. Women dependent on thyroid hormone replacement therapy who are also receiving estrogens may require increased doses of their thyroid replacement therapy. These women should have their thyroid function monitored in order to maintain their free thyroid hormone levels in an acceptable range.
6. Fluid Retention
Estrogens may cause some degree of fluid retention. Women with conditions that might be influenced by this factor, such as a cardiac or renal dysfunction, warrant careful observation when estrogen-alone is prescribed.
7. Hypocalcemia
Estrogen therapy should be used with caution in women with hypoparathyroidism as estrogen- induced hypocalcemia may occur.
8. Exacerbation of Endometriosis
A few cases of malignant transformation of residual endometrial implants have been reported in women treated post-hysterectomy with estrogen alone therapy. For patients known to have residual endometriosis post-hysterectomy, the addition of progestin should be considered.
9. Exacerbation of Other Conditions
Estrogen therapy may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine or porphyria, systemic lupus erythematosus, and hepatic hemangiomas and should be used with caution in women with these conditions.
B. Patient Information
Physicians are advised to discuss the PATIENT INFORMATION leaflet with women for whom they prescribe estradiol vaginal cream USP 0.01%.
C. Laboratory Tests
Serum FSH and estradiol levels have not been shown to be useful in the management of moderate to severe symptoms of vulvar and vaginal atrophy.
D. Drug-Laboratory Test Interactions
- Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex, and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
- Increased thyroid-binding globulin (TBG) leading to increased circulating total thyroid hormone levels, as measured by protein-bound iodine (PBI), T4 levels (by column or by radioimmunoassay) or T3 levels by radioimmunoassay. T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 and free T3 concentrations are unaltered. Women on thyroid replacement therapy may require higher dose of thyroid hormone.
- Other binding proteins may be elevated in serum, i.e., corticosteroid binding globulin (CBG), sex hormone-binding globulin (SHBG), leading to increased total circulating corticosteroids and sex steroids, respectively. Free hormone concentrations, such as testosterone and estradiol, may be decreased. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-antitrypsin, ceruloplasmin).
- Increased plasma high-density lipoprotein (HDL) and HDL2 cholesterol subfraction concentrations, reduced low-density lipoprotein (LDL) cholesterol concentration, increased triglycerides levels.
- Impaired glucose tolerance.
E. Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term continuous administration of estrogen, with and without progestin, in women with and without a uterus, has shown an increased risk of endometrial cancer, breast cancer, and ovarian cancer. (See BOXED WARNINGS, WARNINGS and PRECAUTIONS.)
Long term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver.
F. Pregnancy
Estradiol vaginal cream USP 0.01% should not be used during pregnancy [see CONTRAINDICATIONS].
There appears to be little or no increased risk of birth defects in children born to women who have used estrogens and progestins as an oral contraceptive inadvertently during early pregnancy.
G. Nursing Mothers
Estradiol vaginal cream USP 0.01% should not be used during lactation. Estrogen administration to nursing women has been shown to decrease the quantity and quality of the breast milk. Detectable amounts of estrogens have been identified in the milk of women receiving estrogen therapy. Caution should be exercised when estradiol vaginal cream USP 0.01% is administered to a nursing woman.
H. Pediatric Use
Estradiol vaginal cream USP 0.01% is not indicated in children. Clinical studies have not been conducted in the pediatric population.
I. Geriatric Use
There have not been sufficient numbers of geriatric patients involved in studies utilizing estradiol vaginal cream USP 0.01% to determine whether those over 65 years of age differ from younger subjects in their response to estradiol vaginal cream USP 0.01%.
The Women’s Health Initiative Study
In the WHI estrogen-alone substudy (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age [see CLINICAL STUDIES and WARNINGS].
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age [see CLINICAL STUDIES and WARNINGS].
The Women’s Health Initiative Memory Study
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen-alone or estrogen plus progestin when compared to placebo [see CLINICAL STUDIES and WARNINGS].
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women 10 [see CLINICAL STUDIES and WARNINGS].
- Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex, and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
-
ADVERSE REACTIONS
See BOXED WARNINGS, WARNINGS and PRECAUTIONS.
Systemic absorption may occur with the use of estradiol vaginal cream USP 0.01%. The warnings, precautions, and adverse reactions associated with oral estrogen treatment should be taken into account.
The following adverse reactions have been reported with estrogen and/or progestin therapy.
1. Genitourinary System
Abnormal uterine bleeding or spotting; dysmenorrhea or pelvic pain, increase in size of uterine leiomyomata; vaginitis, including vaginal candidiasis; change in cervical secretion; cystitis-like syndrome; application site reactions of vulvovaginal discomfort including burning and irritation; genital pruritus; ovarian cancer; endometrial hyperplasia; endometrial cancer.
2. Breasts
Tenderness, enlargement, pain, nipple discharge, fibrocystic breast changes; breast cancer.
3. Cardiovascular
Deep and superficial venous thrombosis; pulmonary embolism; myocardial infarction; stroke; increase in blood pressure.
4. Gastrointestinal
Nausea, vomiting; abdominal cramps, bloating; increased incidence of gallbladder disease.
5. Skin
Chloasma that may persist when drug is discontinued; loss of scalp hair; hirsutism; rash.
6. Eyes
Retinal vascular thrombosis, intolerance to contact lenses.
7. Central Nervous System
Headache; migraine; dizziness; mental depression; nervousness; mood disturbances; irritability; dementia.
8. Miscellaneous
Increase or decrease in weight; glucose intolerance; edema; arthralgias; leg cramps; changes in libido; urticaria; exacerbation of asthma; increased triglycerides; hypersensitivity.
-
OVERDOSAGE
Overdosage of estrogen may cause nausea, vomiting, breast tenderness, abdominal pain, drowsiness and fatigue, and withdrawal bleeding may occur in women. Treatment of overdose consists of discontinuation of estradiol vaginal cream USP 0.01% therapy together with institution of appropriate symptomatic care.
-
DOSAGE AND ADMINISTRATION
Use of estradiol vaginal cream USP 0.01% alone or in combination with a progestin, should be limited to the shortest duration consistent with treatment goals and risks for the individual woman. Postmenopausal women should reevaluate periodically as clinically appropriate to determine if treatment is still necessary. For treatment of vulvar and vaginal atrophy associated with the menopause, the lowest dose and regimen that will control symptoms should be chosen and medication should be discontinued as promptly as possible. For women who have a uterus, adequate diagnostic measures, including directed and random endometrial sampling when indicated, should be undertaken to rule out malignancy in cases of undiagnosed persistent or recurring abnormal genital bleeding.
Usual Dosage: The usual dosage range is 2 to 4 g (marked on the applicator) daily for one or two weeks, then gradually reduced to one half initial dosage for a similar period. A maintenance dosage of 1 g, one to three times a week, may be used after restoration of the vaginal mucosa has been achieved.
NOTE: The number of doses per tube will vary with dosage requirements and patient handling.
-
HOW SUPPLIED
Estradiol Vaginal Cream USP 0.01%.
NDC 0093-3541-43: Tube containing 1 ½ oz (42.5 g) with a calibrated plastic applicator for delivery of 1, 2, 3, or 4 g. Smooth semi solid cream. White or off-white.
Store at room temperature 20° to 25°C (59° to 77°F). Protect from temperatures in excess of 40°C (104° F).
Keep Estradiol Vaginal Cream USP 0.01% out of the reach of children.
-
REFERENCES
- Jackson RD, et al. Effects of Conjugated Equine Estrogen on Risk of Fractures and BMD in Postmenopausal Women With Hysterectomy: Results From the Women’s Health Initiative Randomized Trial. J Bone Miner Res. 2006;21:817-828.
- Hendrix SL, et al. Effects of Conjugated Equine Estrogen on Stroke in the Women’s Health Initiative. Circulation. 2006;113:2425-2434.
- Rossouw JE, et al. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause. JAMA. 2007;297:1465-1477.
- Hsia J, et al. Conjugated Equine Estrogens and Coronary Heart Disease. Arch Int Med. 2006;166:357-365.
- Curb JD, et al. Venous Thrombosis and Conjugated Equine Estrogen in Women Without a Uterus. Arch Int Med. 2006;166:772-780.
- Cushman M, et al. Estrogen Plus Progestin and Risk of Venous Thrombosis. JAMA. 2004;292:1573-1580.
- Stefanick ML, et al. Effects of Conjugated Equine Estrogens on Breast Cancer and Mammography Screening in Postmenopausal Women With Hysterectomy. JAMA. 2006;295:1647-1657.
- Chlebowski RT, et al. Influence of Estrogen Plus Progestin on Breast Cancer and Mammography in Healthy Postmenopausal Women. JAMA. 2003;289:3234-3253.
- Anderson GL, et al. Effects of Estrogen Plus Progestin on Gynecologic Cancers and Associated Diagnostic Procedures. JAMA. 2003;290:1739-1748.
- Shumaker SA, et al. Conjugated Equine Estrogens and Incidence of Probable Dementia and Mild Cognitive Impairment in Postmenopausal Women. JAMA. 2004;291:29472958.
Manufactured In Canada by:
Contract Pharmaceuticals Limited
Mississauga, Ontario, Canada L5N 6L6Distributed by:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
1-888-838-2872
© 2024 Teva Pharmaceuticals USA Inc. All rights reserved.
Rev. D 2/2024 - Jackson RD, et al. Effects of Conjugated Equine Estrogen on Risk of Fractures and BMD in Postmenopausal Women With Hysterectomy: Results From the Women’s Health Initiative Randomized Trial. J Bone Miner Res. 2006;21:817-828.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
ESTRADIOL- ESTRADIOL CREAM
(Estradiol Vaginal Cream USP 0.01%)
Read this Patient Information before you start using estradiol vaginal cream USP 0.01% and each time you refill. There may be new information. This information does not take the place of talking with your healthcare provider about your menopausal symptoms or your treatment. What is the most important information I should know about Estradiol Vaginal Cream USP 0.01% (an estrogen hormone)?
- Using estrogen-alone may increase your chance of getting cancer of the uterus (womb). Report any unusual vaginal bleeding right away while you are using estradiol vaginal cream USP 0.01%. Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Have your healthcare provider check any unusual vaginal bleeding to find out the cause.
- Do not use estrogen-alone to prevent heart disease, heart attacks, strokes or dementia (decline in brain function).
- Using estrogen-alone may increase your chances of getting strokes or blood clots.
- Using estrogen-alone may increase your chance of getting dementia, based on a study of women - 65 years of age or older.
- Do not use estrogens with progestins to prevent heart disease, heart attacks, strokes or dementia.
- Using estrogens with progestins may increase your chances of getting heart attacks, strokes, breast cancer, or blood clots.
- Using estrogens with progestins may increase your chance of getting dementia, based on a study of women 65 years of age or older.
- Talk regularly with your healthcare provider about whether you still need treatment with estradiol vaginal cream USP 0.01%.
What is Estradiol Vaginal Cream USP 0.01%?
Estradiol vaginal cream USP 0.01% is a prescription medicine that contains estradiol (an estrogen hormone).What is Estradiol Vaginal Cream USP 0.01% used for?
Estradiol Vaginal Cream USP 0.01% is used after menopause to:-
Treat moderate to severe menopausal changes in and around the vagina.
Talk regularly with your healthcare provider about whether you still need treatment with estradiol vaginal cream USP 0.01%.
Who should not use Estradiol Vaginal Cream USP 0.01%?
Do not start using Estradiol Vaginal Cream USP 0.01% if you:
-
have unusual vaginal bleeding.
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Have your healthcare provider check any unusual bleeding to find out the cause.
-
currently have or have had certain cancers.
Estrogens may increase the chances of getting certain types of cancers, including cancer of the breast or uterus.
If you have or have had cancer, talk with your healthcare provider about whether you should use estradiol vaginal cream USP 0.01%.
-
currently have or have had blood clots.
-
had a stroke or heart attack.
-
are allergic to estradiol vaginal cream USP 0.01% or any of its ingredients.
See the list of ingredients in estradiol vaginal cream USP 0.01% at the end of this leaflet.
-
currently have or have had liver problems.
-
have been diagnosed with a bleeding disorder.
- think you may be pregnant.
Before you use Estradiol Vaginal Cream USP 0.01%, tell your healthcare provider about all of your medical conditions, including if you:
-
have unusual vaginal bleeding.
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
-
have any other medical conditions.
Your healthcare provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing), diabetes, epilepsy (seizures), migraine, endometriosis, lupus, problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood.
-
are going to have surgery or will be on bed rest.
Your healthcare provider will let you know if you need to stop using estradiol vaginal cream USP 0.01%.
-
are breastfeeding.
The hormone in estradiol vaginal cream USP 0.01% can pass into your breast milk.
Tell your healthcare provider about the medicines you take,
including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how estradiol vaginal cream USP 0.01% works. Estradiol vaginal cream USP 0.01% may also affect how your other medicines work. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.How should I use Estradiol Vaginal Cream USP 0.01%?
Estradiol vaginal cream USP 0.01% is a cream that you place in your vagina with the applicator provided with the cream.
- Take the dose recommended by your healthcare provider and talk to them about how well that dose is working for you.
- Use estrogens at the lowest dose possible for your treatment only as long as needed. Talk regularly (for example, every 3 to 6 months) with your healthcare provider about the dose you are using and whether you still need treatment with estradiol vaginal cream USP 0.01%.
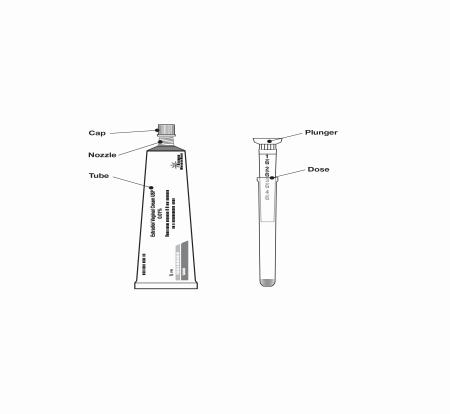
Figure A
Step 1 Wash and dry your hands well.
Step 2. Remove the cap from the estradiol vaginal cream USP 0.01% tube. (There is no seal on tube).
Step 3. Hold the applicator as shown. Do not separate the plunger from applicator (Figure B).
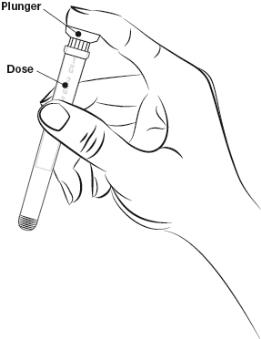
Figure B
Step 4. Screw threaded end of applicator onto the open nozzle of the estradiol vaginal cream USP 0.01% tube until secure. Do not attach the plunger end of the applicator onto the open estradiol vaginal cream USP 0.01% tube (see Figure C).
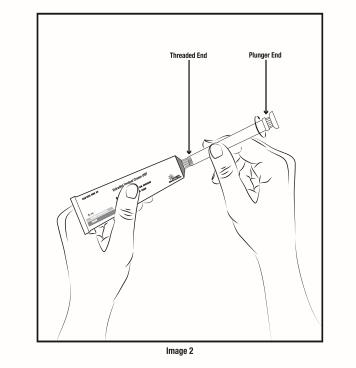
Figure C
Step 5. Position upright to view the calibrated gram amounts.
Step 6. Gently squeeze tube from the bottom to push the prescribed amount of
estradiol vaginal cream USP 0.01% into the applicator. As estradiol vaginal cream USP 0.01% is squeezed out, the plunger will rise to indicate the amount of grams (see Figure D).
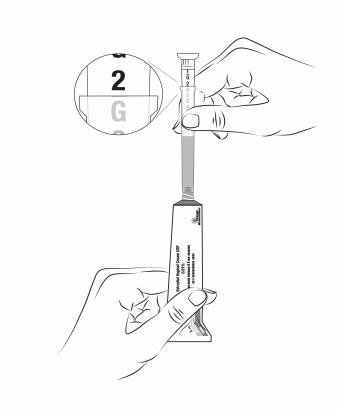
Figure D
*This Figure shows a 2 g dose for illustrative purposes only. Your prescribed dosage may vary.
Step 7. Unscrew applicator from tube.
Step 8. Replace cap on tube.
Step 9 Lie on your back with knees bent. To deliver the medicine, gently insert applicator deeply into your vagina and press the plunger downward to its original position (see Figure E).
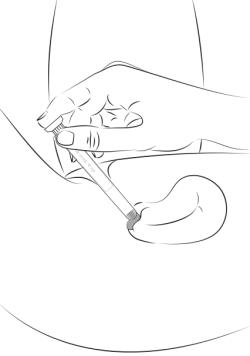
Figure E
Step 10 Remove the applicator from your vagina.
Step 11. To cleanse applicator: Pull plunger to remove it from barrel. Wash with mild soap and warm water (Do not boil or use hot water) (see Figure F).
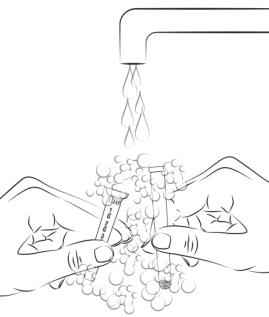
Figure F
What are the possible side effects of Estradiol Vaginal Cream USP 0.01%?
Side effects are grouped by how serious they are and how often they happen when you are treated.
Serious, but less common side effects include:
- stroke
- blood clots
- breast cancer
- dementia
- high blood calcium (hypercalcemia)
- severe allergic reaction
- high blood pressure
- liver problems
- fluid retention
- worsening of endometriosis
- changes in certain laboratory test results
- heart disease
- cancer of the lining of the uterus (womb)
- cancer of the ovary
- gallbladder disease
- changes in vision
- worsening of angioedema (swelling of face and tongue)
- high triglyceride (fat) levels in your blood
- low thyroid levels in your blood
- low blood calcium (hypocalcemia)
- worsening of other medical conditions
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- new breast lumps
- unusual vaginal bleeding
- changes in vision or speech
- sudden new severe headaches
- severe pains in your chest or legs with or without shortness of breath, weakness and fatigue
- swollen lips, tongue or face
- headache
- breast pain
- irregular vaginal bleeding or spotting
- stomach or abdominal cramps, bloating
- nausea and vomiting
- hair loss
- fluid retention
- vaginal yeast infection
- reactions from inserting estradiol vaginal cream USP 0.01%, such as vaginal burning, irritation, and itching
How should I store Estradiol vaginal cream USP 0.01%? -
Store Estradiol Vaginal Cream USP 0.01% at room temperature between 59°F to 77°F (20°C to 25°C)
-
Protect from temperatures above 104°F (40°C)
- Keep Estradiol Vaginal Cream USP 0.01% and all medicines out of the reach of children
What can I do to lower my chances of a serious side effect with Estradiol Vaginal Cream USP 0.01%?
- Talk with your healthcare provider regularly about whether you should continue using estradiol vaginal cream USP 0.01%.
- If you have a uterus, talk with your healthcare provider about whether the addition of a progestin is right for you. In general, the addition of a progestin is generally recommended for a woman with a uterus to reduce the chance of getting cancer of the uterus.
- See your healthcare provider right away if you get vaginal bleeding while using estradiol vaginal cream USP 0.01%.
- Have a pelvic exam, breast exam and mammogram (breast X-ray) every year unless your healthcare provider tells you something else. If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram, you may need to have breast exams more often.
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have higher chances for getting heart disease. Ask your healthcare provider for ways to lower your chances for getting heart disease.
General information about safe and effective use of Estradiol Vaginal Cream USP 0.01%
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use estradiol vaginal cream USP 0.01% for conditions for which it was not prescribed. Do not give estradiol vaginal cream USP 0.01% to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about estradiol vaginal cream USP 0.01% that is written for health professionals.What are the ingredients in Estradiol Vaginal Cream USP 0.01%?
Active ingredient: estradiol
Inactive Ingredients: purified water, propylene glycol, stearyl alcohol, white ceresin wax, mono- and di- glycerides, hypromellose 2208 (4000 cps), sodium lauryl sulfate, methylparaben, edetate di- sodium and tertiary-butylhydroquinone.Manufactured In Canada by:
Contract Pharmaceuticals Limited
Mississauga, Ontario, Canada L5N 6L6
Distributed by:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
1-888-838-2872
© 2024 Teva Pharmaceuticals USA Inc. All rights reserved.
Rev. D 2/2024 - Using estrogen-alone may increase your chance of getting cancer of the uterus (womb). Report any unusual vaginal bleeding right away while you are using estradiol vaginal cream USP 0.01%. Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Have your healthcare provider check any unusual vaginal bleeding to find out the cause.
-
PRINCIPAL DISPLAY PANEL
NDC 0093-3541-43
Estradiol Vaginal Cream USP
0.01%
UNSCENTED
Each gram contains 0.1 mg estradiol in a nonliquefying base.
Usual Dosage: See enclosed Package Insert for Full Prescribing Information.
CAUTION: Keep this and all medications out of the reach of children.
CALIBRATED APPLICATOR ENCLOSED
This product also contains purified water, propylene glycol, stearyl alcohol, white ceresin wax, mono- and di-glycerides, hypromellose, sodium lauryl sulfate, methylparaben, edetate disodium, and t-butylhydroquinone.
Read Accompanying Information For The Patient
SHAPING
WOMEN’S HEALTH®
Rx only
NET WT 1 ½ OZ (42.5 G) TUBE
-
INGREDIENTS AND APPEARANCE
ESTRADIOL
estradiol creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0093-3541 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 0.1 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) CERESIN (UNII: Q1LS2UJO3A) GLYCERYL CAPRYLOCAPRATE (UNII: U72Q2I8C85) HYPROMELLOSE 2208 (4000 MPA.S) (UNII: 39J80LT57T) SODIUM LAURYL SULFATE (UNII: 368GB5141J) METHYLPARABEN (UNII: A2I8C7HI9T) EDETATE DISODIUM (UNII: 7FLD91C86K) TERT-BUTYLHYDROQUINONE (UNII: C12674942B) Product Characteristics Color white (White or off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0093-3541-43 1 in 1 CARTON 01/02/2018 1 42.5 g in 1 TUBE, WITH APPLICATOR; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA086069 01/02/2018 Labeler - Teva Pharmaceuticals USA, Inc. (001627975)