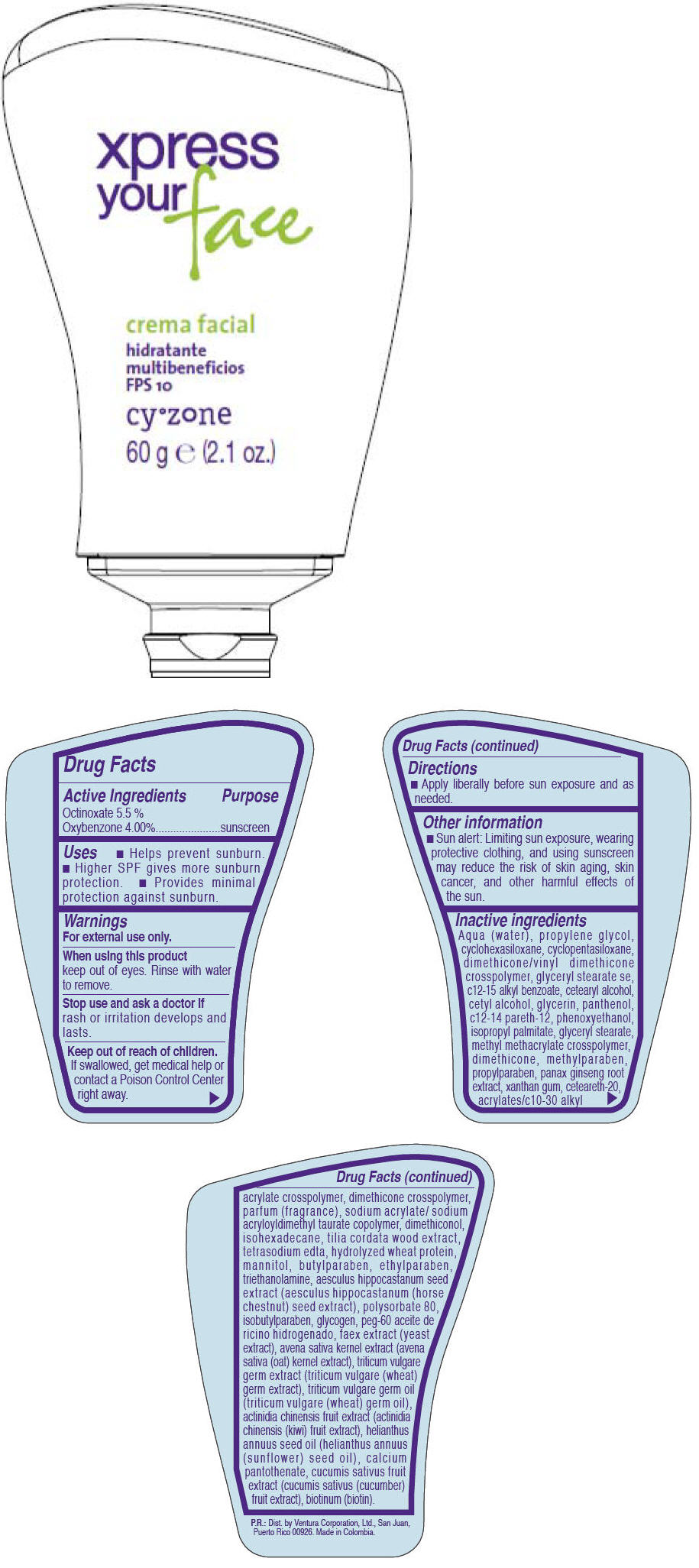
Label: CYZONE- octinoxate and oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-356-01 - Packager: Ventura Corporation (San Juan, P.R)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 26, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Other information
-
Inactive ingredients
AQUA (WATER), PROPYLENE GLYCOL, CYCLOHEXASILOXANE, CYCLOPENTASILOXANE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, GLYCERYL STEARATE SE, C12-15 ALKYL BENZOATE, CETEARYL ALCOHOL, CETYL ALCOHOL, GLYCERIN, PANTHENOL, C12-14 PARETH-12, PHENOXYETHANOL, ISOPROPYL PALMITATE, GLYCERYL STEARATE, METHYL METHACRYLATE CROSSPOLYMER, DIMETHICONE, METHYLPARABEN, PROPYLPARABEN, PANAX GINSENG ROOT EXTRACT, XANTHAN GUM, CETEARETH-20, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, DIMETHICONE CROSSPOLYMER, PARFUM (FRAGRANCE), SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, DIMETHICONOL, ISOHEXADECANE, TILIA CORDATA WOOD EXTRACT, TETRASODIUM EDTA, HYDROLYZED WHEAT PROTEIN, MANNITOL, BUTYLPARABEN, ETHYLPARABEN, TRIETHANOLAMINE, AESCULUS HIPPOCASTANUM SEED EXTRACT (AESCULUS HIPPOCASTANUM (HORSE CHESTNUT) SEED EXTRACT), POLYSORBATE 80, ISOBUTYLPARABEN, GLYCOGEN, PEG-60 ACEITE DE RICINO HIDROGENADO, FAEX EXTRACT (YEAST EXTRACT), AVENA SATIVA KERNEL EXTRACT (AVENA SATIVA (OAT) KERNEL EXTRACT), TRITICUM VULGARE GERM EXTRACT (TRITICUM VULGARE (WHEAT) GERM EXTRACT), TRITICUM VULGARE GERM OIL (TRITICUM VULGARE (WHEAT) GERM OIL), ACTINIDIA CHINENSIS FRUIT EXTRACT (ACTINIDIA CHINENSIS (KIWI) FRUIT EXTRACT), HELIANTHUS ANNUUS SEED OIL (HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL), CALCIUM PANTOTHENATE, CUCUMIS SATIVUS FRUIT EXTRACT (CUCUMIS SATIVUS (CUCUMBER) FRUIT EXTRACT), BIOTINUM (BIOTIN)
- PRINCIPAL DISPLAY PANEL - 60 g Bottle
-
INGREDIENTS AND APPEARANCE
CYZONE EXPRESS YOUR FACE
octinoxate and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-356 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.055 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.04 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) PANTHENOL (UNII: WV9CM0O67Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) DIMETHICONE (UNII: 92RU3N3Y1O) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ASIAN GINSENG (UNII: CUQ3A77YXI) XANTHAN GUM (UNII: TTV12P4NEE) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) ISOHEXADECANE (UNII: 918X1OUF1E) TILIA CORDATA WOOD (UNII: 674MYE89IE) EDETATE SODIUM (UNII: MP1J8420LU) MANNITOL (UNII: 3OWL53L36A) BUTYLPARABEN (UNII: 3QPI1U3FV8) ETHYLPARABEN (UNII: 14255EXE39) TROLAMINE (UNII: 9O3K93S3TK) HORSE CHESTNUT (UNII: 3C18L6RJAZ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) GLYCOGEN (UNII: 309GSC92U1) YEAST (UNII: 3NY3SM6B8U) OAT (UNII: Z6J799EAJK) WHEAT GERM (UNII: YR3G369F5A) WHEAT GERM OIL (UNII: 14C97E680P) KIWI FRUIT (UNII: 71ES77LGJC) SUNFLOWER OIL (UNII: 3W1JG795YI) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) CUCUMBER (UNII: YY7C30VXJT) BIOTIN (UNII: 6SO6U10H04) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-356-01 60 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2011 Labeler - Ventura Corporation (San Juan, P.R) (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE