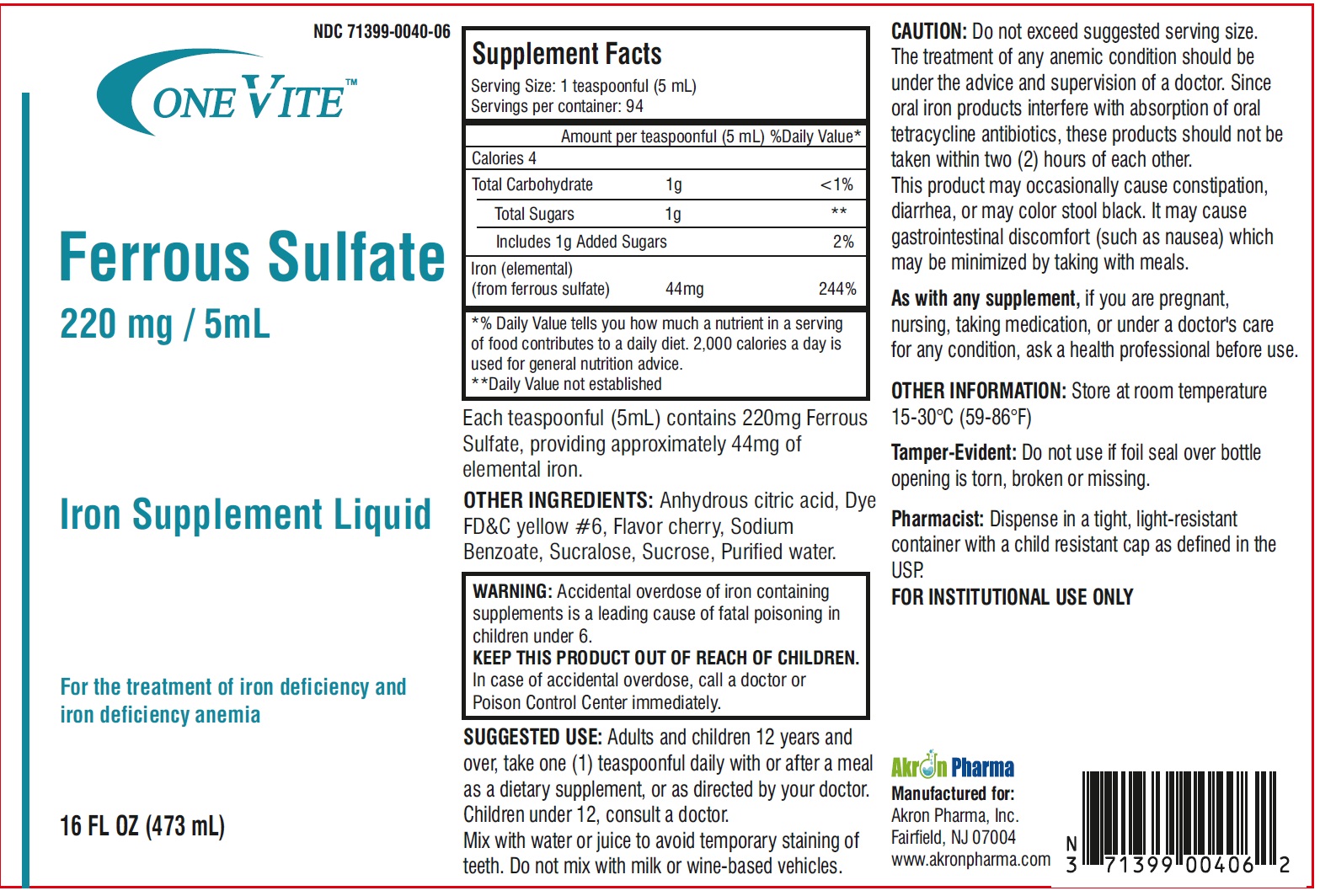
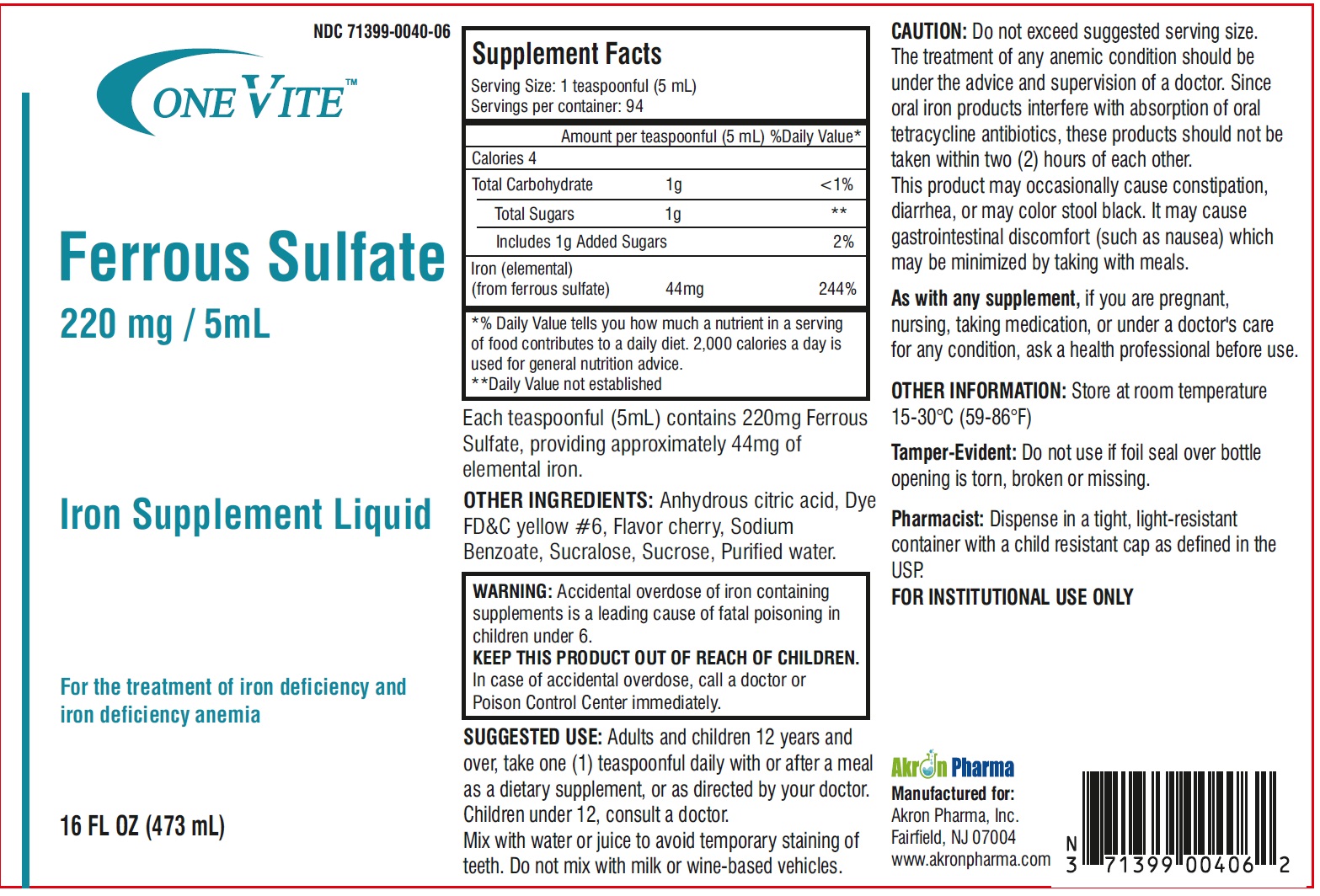
Label: ONE VITE IRON SUPPLEMENT LIQUID- ferrous sulfate liquid
- NHRIC Code(s): 71399-0040-6
- Packager: Akron Pharma
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated August 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
Supplement Facts Serving Size: 1 teaspoonful (5 mL)
Servings per container: 94Amount per teaspoonful (5 mL) %Daily Value * % Daily Value Calories 4 Total Carbohydrate 1g <1% Total Sugars 1g ** includes 1g Added Sugars 2% Iron (elemental)
(from ferrous sulfate) 44mg244% *% Daily Value tells you how much a nutrient in a serving
of food contributes to a daily diet. 2,000 calories a day is
used for general nutrition advice.
**Daily Value not established - HEALTH CLAIM
- SAFE HANDLING WARNING
- WARNINGS
-
PRECAUTIONS
CAUTION: Do not exceed suggested serving size.
The treatment of any anemic condition should be under the advice and supervision of a doctor. Since oral iron products interfere with absorption of oral tetracycline antibiotics, these products should not be taken within two (2) hours of each other.
This product may occasionally cause constipation, diarrhea, or may color stool black. It may cause gastrointestinal discomfort (such as nausea) which may be minimized by taking with meals.
As with any supplement, if you are pregnant, nursing, taking medication, or under a doctor's care for any condition, ask a health professional before use.
OTHER INFORMATION: Store at room temperature 15-30°C (59-86°F)Tamper-Evident: Do not use if foil seal over bottle opening is torn, broken or missing.
Pharmacist: Dispense in a tight, light-resistant container with a child resistant cap as defined in the USP. -
DOSAGE & ADMINISTRATION
SUGGESTED USE: Adults and children 12 years and over, take one (1) teaspoonful daily with or after a meal as a dietary supplement, or as directed by your doctor.
Children under 12, consult a doctor.
Mix with water or juice to avoid temporary staining of teeth. Do not mix with milk or wine-based vehicles.Manufactured for:
Akron Pharma, Inc.
Fairfield, NJ 07004
www.akronpharma.com - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ONE VITE IRON SUPPLEMENT LIQUID
ferrous sulfate liquidProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:71399-0040 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERROUS SULFATE (UNII: 39R4TAN1VT) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 220 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:71399-0040-6 473 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 08/04/2023 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value flavor Labeler - Akron Pharma (067878881)