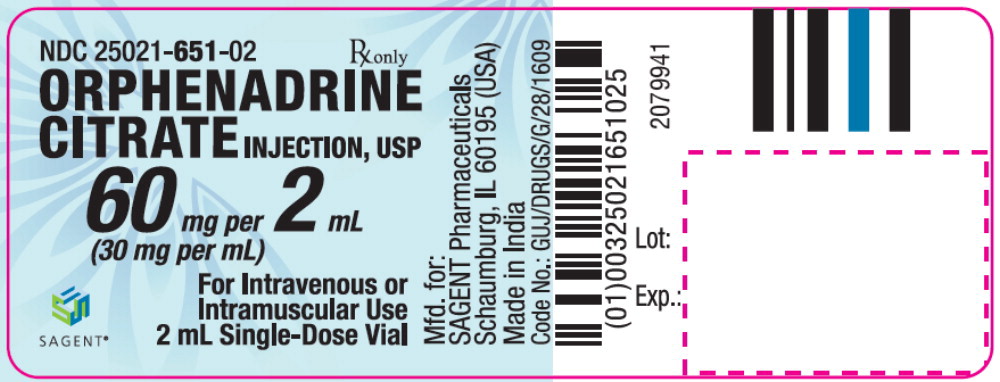
Label: ORPHENADRINE CITRATE injection, solution
- NDC Code(s): 25021-651-02
- Packager: Sagent Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
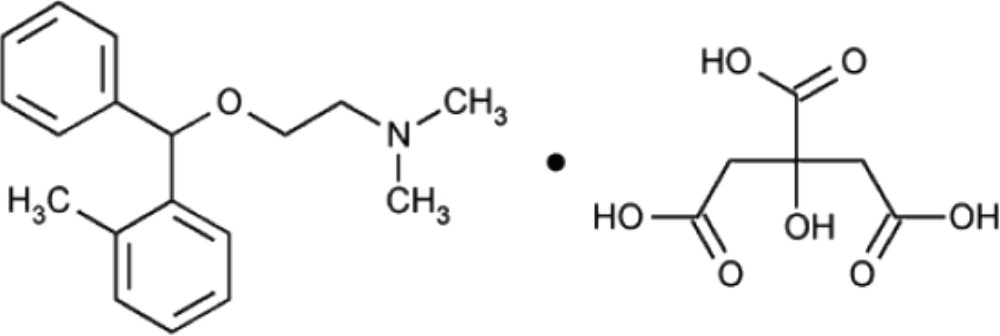
Orphenadrine citrate is the citrate salt of orphenadrine (2-dimethylaminoethyl 2- methylbenzhydryl ether citrate). It occurs as a white, crystalline powder having a bitter taste. It is practically odorless; sparingly soluble in water, slightly soluble in alcohol.
It has the following structural formula:The empirical formula is C18H23NO·C6H8O7, representing a molecular weight of 461.50.
Orphenadrine Citrate Injection, USP contains 60 mg of orphenadrine citrate in aqueous solution in each vial. Orphenadrine Citrate Injection, USP also contains: sodium metabisulfite NF, 2.0 mg; sodium chloride USP, 5.8 mg; sodium hydroxide NF, to adjust pH; and water for injection USP, q.s. to 2 mL.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Contraindicated in patients with glaucoma, pyloric or duodenal obstruction, stenosing peptic ulcers, prostatic hypertrophy or obstruction of the bladder neck, cardio-spasm (megaesophagus) and myasthenia gravis.
Contraindicated in patients who have demonstrated a previous hypersensitivity to the drug.
-
WARNINGS
Some patients may experience transient episodes of light-headedness, dizziness or syncope. Orphenadrine citrate may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; ambulatory patients should therefore be cautioned accordingly.
Orphenadrine citrate contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than nonasthmatic people.
-
PRECAUTIONS
Confusion, anxiety and tremors have been reported in few patients receiving propoxyphene and orphenadrine concomitantly. As these symptoms may be simply due to an additive effect, reduction of dosage and/or discontinuation of one or both agents is recommended in such cases.
Orphenadrine citrate should be used with caution in patients with tachycardia, cardiac decompensation, coronary insufficiency, cardiac arrhythmias.
Safety of continuous long-term therapy with orphenadrine has not been established. Therefore, if orphenadrine is prescribed for prolonged use, periodic monitoring of blood, urine and liver function values is recommended.
Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with orphenadrine citrate It is also not known whether orphenadrine citrate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Orphenadrine citrate should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
Adverse reactions of orphenadrine are mainly due to the mild anti-cholinergic action of orphenadrine, and are usually associated with higher dosage. Dryness of the mouth is usually the first adverse effect to appear. When the daily dose is increased, possible adverse effects include: tachycardia, palpitation, urinary hesitancy or retention, blurred vision, dilatation of pupils, increased ocular tension, weakness, nausea, vomiting, headache, dizziness, constipation, drowsiness, hypersensitivity reactions, pruritus, hallucinations, agitation, tremor, gastric irritation, and rarely urticaria and other dermatoses. Infrequently, an elderly patient may experience some degree of mental confusion. These adverse reactions can usually be eliminated by reduction in dosage. Very rare cases of aplastic anemia associated with the use of orphenadrine tablets have been reported. No causal relationship has been established.
Rare instances of anaphylactic reaction have been reported associated with the intramuscular injection of orphenadrine citrate.
To report SUSPECTED ADVERSE REACTIONS, contact Sagent Pharmaceuticals, Inc. at 1-866-625-1618 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
Orphenadrine is toxic when overdosed and typically induces anticholinergic effects. In a review of orphenadrine toxicity, the minimum lethal dose was found to be 2 to 3 grams for adults; however, the range of toxicity is variable and unpredictable. Treatment for orphenadrine overdose is evacuation of stomach contents (when necessary), charcoal at repeated doses, intensive monitoring, and appropriate supportive treatment of any emergent anticholinergic effects.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Orphenadrine Citrate Injection, USP is supplied as follows:
NDC Orphenadrine Citrate Injection, USP (30 mg per mL) Package Factor 25021-651-02 60 mg per 2 mL Single-Dose Vial 10 vials per carton Orphenadrine Citrate Injection, USP is an aqueous solution.
Storage Conditions
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F). [See USP Controlled Room Temperature.]
Protect from light. Retain in carton until time of use.
Not to be sold in unbroken box.
Discard unused portion.
Sterile, Nonpyrogenic, Preservative-free.
The container closure is not made with natural rubber latex.SAGENT®
Mfd. for SAGENT Pharmaceuticals
Schaumburg, IL 60195 (USA)
Made in India
©2021 Sagent Pharmaceuticals, Inc.Revised: May 2021
SAGENT Pharmaceuticals®
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ORPHENADRINE CITRATE
orphenadrine citrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25021-651 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength orphenadrine citrate (UNII: X0A40N8I4S) (orphenadrine - UNII:AL805O9OG9) orphenadrine citrate 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength sodium metabisulfite (UNII: 4VON5FNS3C) 1 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) 2.9 mg in 1 mL sodium hydroxide (UNII: 55X04QC32I) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25021-651-02 10 in 1 CARTON 10/01/2011 1 2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090585 10/01/2011 Labeler - Sagent Pharmaceuticals (080579617)