Label: SENNA- sennosides syrup
-
Contains inactivated NDC Code(s)
NDC Code(s): 69618-069-58 - Packager: Reliable 1 Laboratories LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 3, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
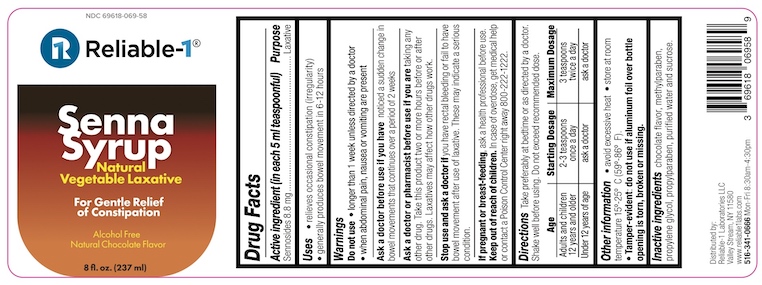
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not use
- longer than 1 week unless directed by a doctor
- when abdominal pain, nausea or vomiting are present
Ask a doctor before use if you have noticed a sudden change in bowel movements that continues over a period of 2 weeks
Ask a doctor or pharmacist before use if you are taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SENNA
sennosides syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69618-069 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.8 mg in 5 mL Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color brown Score Shape Size Flavor CHOCOLATE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69618-069-58 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/03/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 08/03/2021 Labeler - Reliable 1 Laboratories LLC (079718111)