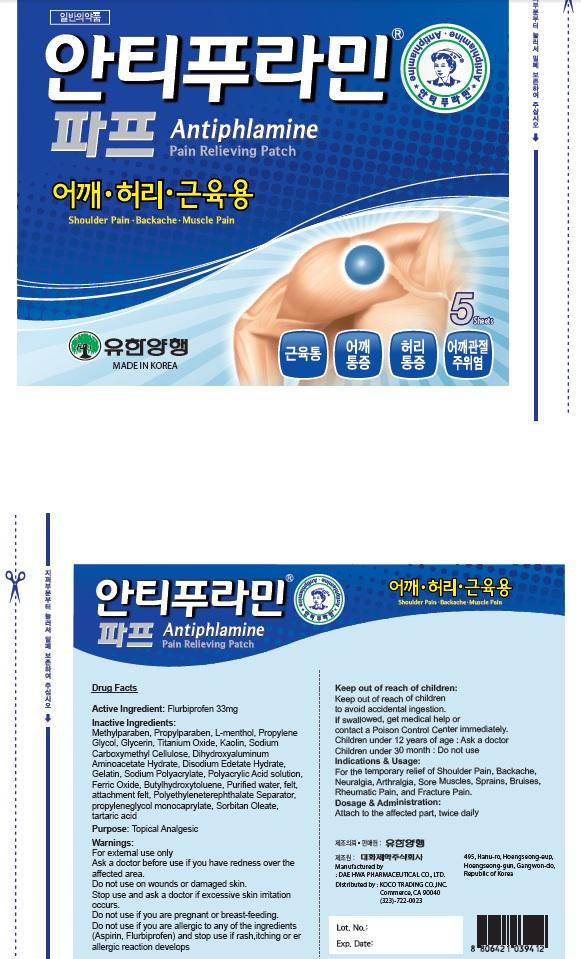
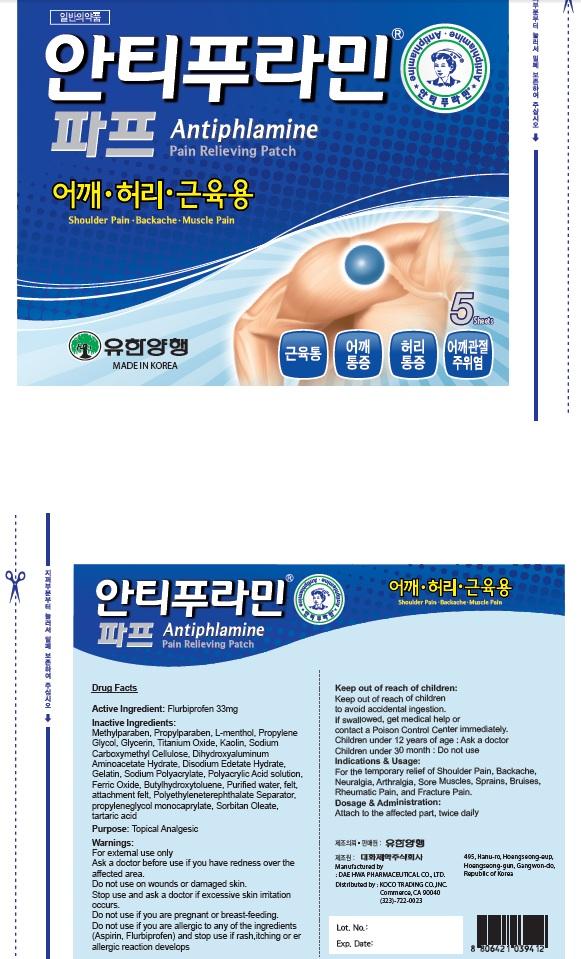
Label: ANTIPHLAMINE PAIN RELIEVING- flurbiprofen patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 69642-1300-1 - Packager: HANUL TRADING CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 13, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive Ingredients: Methylparaben, Propylparaben, L-menthol, Propylene Glycol, Glycerin, Titanium Oxide, Kaolin, Sodium Carboxymethyl Cellulose, Dihydroxyaluminum Aminoacetate Hydrate, Disodium Edetate Hydrate, Gelatin, Sodium Polyacrylate, Polyacrylic Acid solution, Ferric Oxide, Butylhydroxytoluene, Purified water, felt, attachment felt, Polyethyleneterephthalate Separator, propyleneglycol monocaprylate, Sorbitan Oleate, tartaric acid
- PURPOSE
-
WARNINGS
Warnings: For external use only Ask a doctor before use if you have redness over the affected area. Do not use: on wounds or damaged skin. Stop use and ask a doctor if excessive skin irritation occurs. Do not use if you are pregnant or breast feading. Do not use if you are allergic to any of the ingredients(Aspirin, Flurbiprofen) and stop use if rash, itching or allergic reaction develops.
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTIPHLAMINE PAIN RELIEVING
flurbiprofen patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69642-1300 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Flurbiprofen (UNII: 5GRO578KLP) (FLURBIPROFEN - UNII:5GRO578KLP) Flurbiprofen 33 mg in 5 Inactive Ingredients Ingredient Name Strength Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69642-1300-1 5 in 1 CELLO PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/01/2015 Labeler - HANUL TRADING CO., LTD. (689512982) Registrant - HANUL TRADING CO., LTD. (689512982) Establishment Name Address ID/FEI Business Operations HANUL TRADING CO., LTD. 689512982 repack(69642-1300) Establishment Name Address ID/FEI Business Operations Dae Hwa Pharm Co., Ltd. 688004324 manufacture(69642-1300) Establishment Name Address ID/FEI Business Operations KOCO TRADING CO., INC. 079457993 wholesale drug distributor(69642-1300)