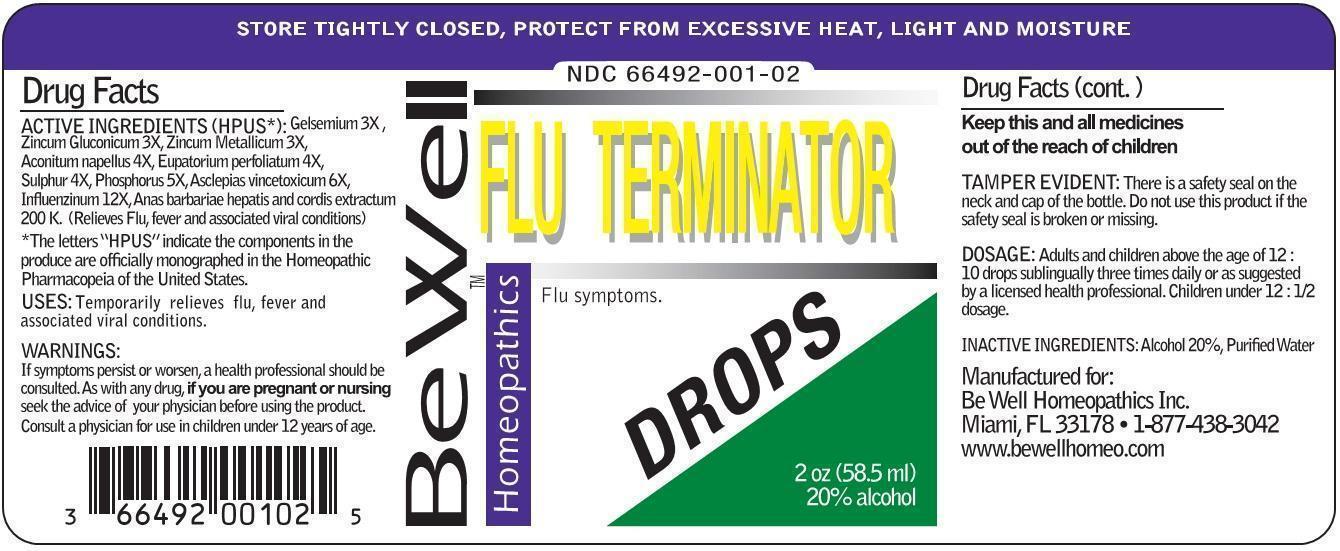
Label: FLU TERMINATOR DROPS- gelsemium, zincum gluconicum, zincum metallicum, aconitum napellus, eupatorium perfoliatum, sulphur, phosphorus, asclepias vincetoxicum, influenzinum, anas barbariae. liquid
- NDC Code(s): 66492-001-02
- Packager: Be Well Medical dba Richard Clement Nutrition y Be Well Homeopathics
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
ACTIVE INGREDIENTS (HPUS*): Gelsemium 3X, Zincum gluconicum 3X, Zincum metallicum 3X, Aconitum napellus 4X, Eupatorium perfoliatum 4X, Sulphur 4X, Phosphorus 5X, Asclepias vincetoxinum 6X, Influenzinum 12X, Anas barbarie hepatis et cordis extractum 200K. (Relieves flu, fever and associated viral conditions)
*The letters "HPUS" indicate the components in the produce are officially monographed in the Homeopathic Pharmacopeia of the United States.
- INDICATIONS & USAGE
- WARNINGS
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLU TERMINATOR DROPS
gelsemium, zincum gluconicum, zincum metallicum, aconitum napellus, eupatorium perfoliatum, sulphur, phosphorus, asclepias vincetoxicum, influenzinum, anas barbariae. liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66492-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 3 [hp_X] in 1 mL ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) ZINC GLUCONATE 3 [hp_X] in 1 mL ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 3 [hp_X] in 1 mL ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 4 [hp_X] in 1 mL EUPATORIUM PERFOLIATUM FLOWERING TOP (UNII: 1W0775VX6E) (EUPATORIUM PERFOLIATUM FLOWERING TOP - UNII:1W0775VX6E) EUPATORIUM PERFOLIATUM FLOWERING TOP 4 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 4 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 5 [hp_X] in 1 mL CYNANCHUM VINCETOXICUM ROOT (UNII: 9R858U917W) (CYNANCHUM VINCETOXICUM ROOT - UNII:9R858U917W) CYNANCHUM VINCETOXICUM ROOT 6 [hp_X] in 1 mL INFLUENZA A VIRUS (UNII: R9HH0NDE2E) (INFLUENZA A VIRUS - UNII:R9HH0NDE2E) INFLUENZA A VIRUS 12 [hp_X] in 1 mL INFLUENZA B VIRUS (UNII: 1314JZ2X6W) (INFLUENZA B VIRUS - UNII:1314JZ2X6W) INFLUENZA B VIRUS 12 [hp_X] in 1 mL CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE (UNII: RN2HC612GY) (CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE - UNII:RN2HC612GY) CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE 200 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66492-001-02 58.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 01/01/2000 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/2000 Labeler - Be Well Medical dba Richard Clement Nutrition y Be Well Homeopathics (052584997)