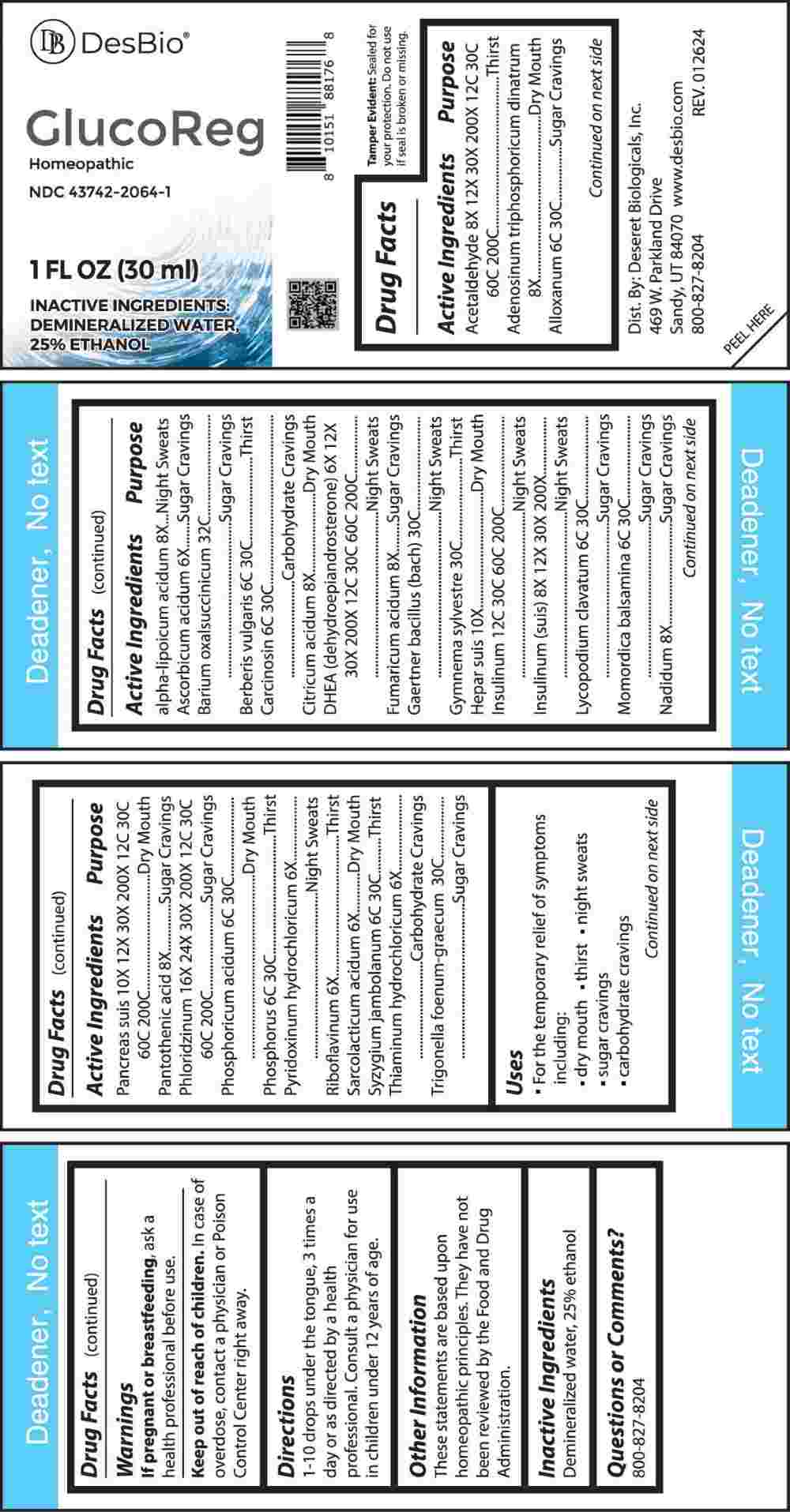
Label: GLUCOREG (ascorbicum acidum, pyridoxinum hydrochloricum, riboflavinum, sarcolacticum acidum, thiaminum hydrochloricum, dhea (dehydroepiandrosterone), adenosinum triphosphoricum dinatrum, alpha-lipoicum acidum, citricum acidum, fumaricum acidum, nadidum, pantothenic acid, acetaldehyde, insulinum (suis), insulinum- human, hepar suis, pancreas suis, phloridzinum, alloxanum, berberis vulgaris, carcinosin, lycopodium clavatum, momordica balsamina, phosphoricum acidum, phosphorus, syzygium jambolanum, liquid
- NDC Code(s): 43742-2064-1
- Packager: Deseret Biologicals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS:
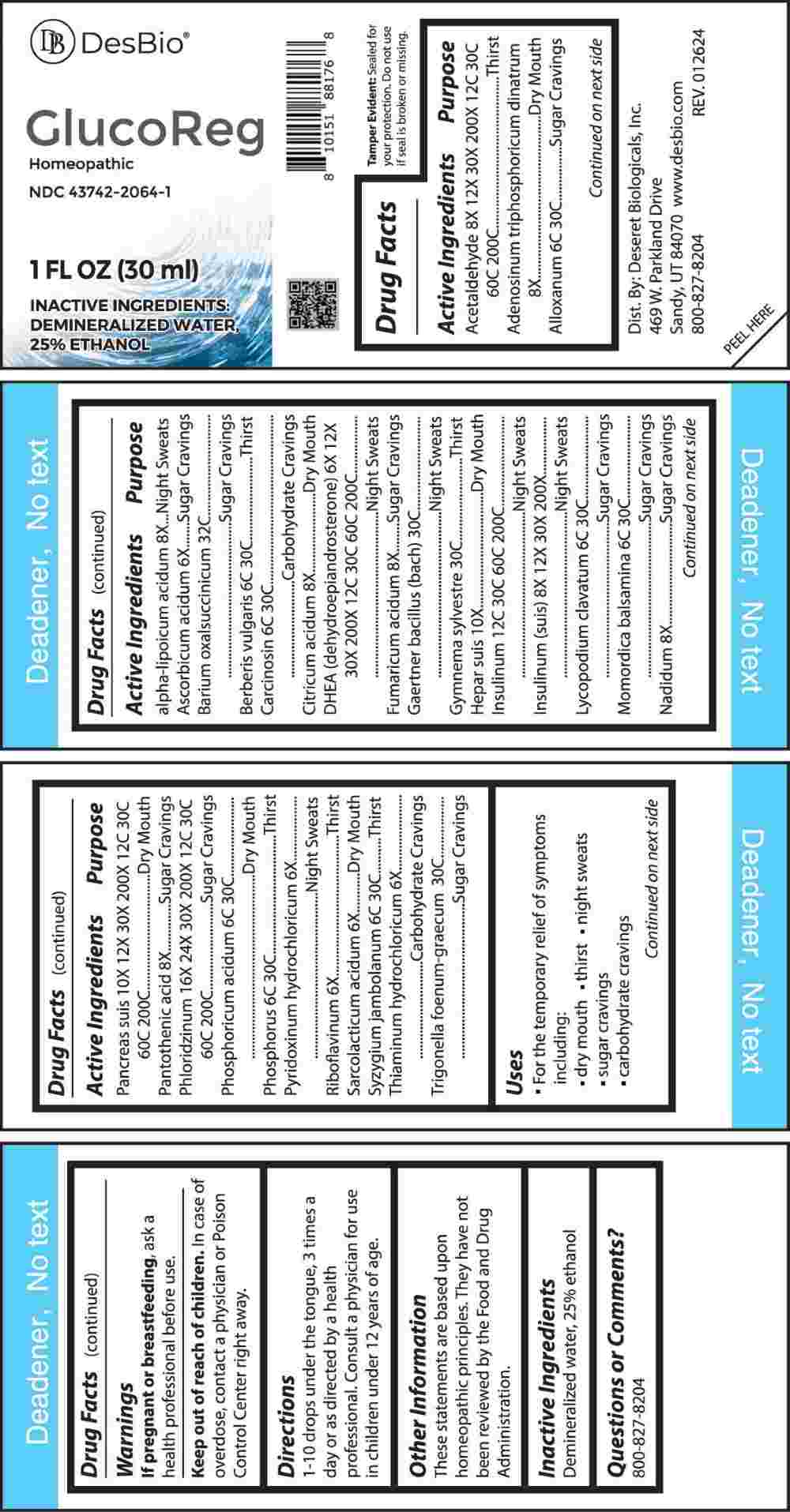
Acetaldehyde 8X, 12X, 30X, 200X, 12C, 30C, 60C, 200C, Adenosinum Triphosphoricum Dinatrum 8X, Alloxanum 6C, 30C, Alpha-Lipoicum Acidum 8X, Ascorbic Acid 6X, Barium Oxalsuccinicum 32C, Berberis Vulgaris 6C, 30C, Carcinosin 6C, 30C, Citricum Acidum 8X, DHEA (Dehydroepiandrosterone) 6X, 12X, 30X, 200X, 12C, 30C, 60C, 200C, Fumaricum Acidum 8X, Gaertner Bacillus (Bach) 30C, Gymnema Sylvestre 30C, Hepar Suis 10X, Insulinum 12C, 30C, 60C, 200C, Insulinum (Suis) 8X, 12X, 30X, 200X, Lycopodium Clavatum 6C, 30C, Momordica Balsamina 6C, 30C, Nadidum 8X, Pancreas Suis 10X, 12X, 30X, 200X, 12C, 30C, 60C, 200C, Pantothenic Acid 8X, Phloridzinum 16X, 24X, 30X, 200X, 12C, 30C, 60C, 200C, Phosphoricum Acidum 6C, 30C, Phosphorus 6C, 30C, Pyridoxinum Hydrochloricum 6X, Riboflavinum 6X, Sarcolacticum Acidum 6X, Syzygium Jambolanum 6C, 30C, Thiaminum Hydrochloricum 6X, Trigonella Foenum-Graecum 30C.
-
PURPOSE:
Acetaldehyde - Thirst, Adenosinum Triphosphoricum Dinatrum – Dry Mouth, Alloxanum – Sugar Cravings, Alpha-Lipoicum Acidum – Night Sweats, Ascorbic Acid – Sugar Cravings, Barium Oxalsuccinicum – Sugar Cravings, Berberis Vulgaris - Thirst, Carcinosin – Carbohydrate Cravings, Citricum Acidum – Dry Mouth, DHEA (Dehydroepiandrosterone) – Night Sweats, Fumaricum Acidum – Sugar Cravings, Gaertner Bacillus (Bach) – Night Sweats, Gymnema Sylvestre - Thirst, Hepar Suis – Dry Mouth, Insulinum – Night Sweats, Insulinum (Suis) – Night Sweats, Lycopodium Clavatum – Sugar Cravings, Momordica Balsamina – Sugar Cravings, Nadidum – Sugar Cravings, Pancreas Suis – Dry Mouth, Pantothenic Acid – Sugar Cravings, Phloridzinum – Sugar Cravings, Phosphoricum Acidum – Dry Mouth, Phosphorus - Thirst, Pyridoxinum Hydrochloricum – Night Sweats, Riboflavinum - Thirst, Sarcolacticum Acidum – Dry Mouth, Syzygium Jambolanum - Thirst, Thiaminum Hydrochloricum – Carbohydrate Cravings, Trigonella Foenum-Graecum – Sugar Cravings
- USES:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
GLUCOREG
ascorbicum acidum, pyridoxinum hydrochloricum, riboflavinum, sarcolacticum acidum, thiaminum hydrochloricum, dhea (dehydroepiandrosterone), adenosinum triphosphoricum dinatrum, alpha-lipoicum acidum, citricum acidum, fumaricum acidum, nadidum, pantothenic acid, acetaldehyde, insulinum (suis), insulinum (human), hepar suis, pancreas suis, phloridzinum, alloxanum, berberis vulgaris, carcinosin, lycopodium clavatum, momordica balsamina, phosphoricum acidum, phosphorus, syzygium jambolanum, liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43742-2064 Route of Administration Oral Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 6 [hp_X] in 1 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 6 [hp_X] in 1 mL RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 6 [hp_X] in 1 mL LACTIC ACID, L- (UNII: F9S9FFU82N) (LACTIC ACID, L- - UNII:F9S9FFU82N) LACTIC ACID, L- 6 [hp_X] in 1 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 6 [hp_X] in 1 mL PRASTERONE (UNII: 459AG36T1B) (PRASTERONE - UNII:459AG36T1B) PRASTERONE 6 [hp_X] in 1 mL ADENOSINE TRIPHOSPHATE DISODIUM (UNII: 5L51B4DR1G) (ADENOSINE TRIPHOSPHATE - UNII:8L70Q75FXE) ADENOSINE TRIPHOSPHATE DISODIUM 8 [hp_X] in 1 mL THIOCTIC ACID (UNII: 73Y7P0K73Y) (.ALPHA.-LIPOIC ACID - UNII:73Y7P0K73Y) THIOCTIC ACID 8 [hp_X] in 1 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 8 [hp_X] in 1 mL FUMARIC ACID (UNII: 88XHZ13131) (FUMARIC ACID - UNII:88XHZ13131) FUMARIC ACID 8 [hp_X] in 1 mL NADIDE (UNII: 0U46U6E8UK) (NADIDE - UNII:0U46U6E8UK) NADIDE 8 [hp_X] in 1 mL PANTOTHENIC ACID (UNII: 19F5HK2737) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 8 [hp_X] in 1 mL ACETALDEHYDE (UNII: GO1N1ZPR3B) (ACETALDEHYDE - UNII:GO1N1ZPR3B) ACETALDEHYDE 8 [hp_X] in 1 mL INSULIN PORK (UNII: AVT680JB39) (INSULIN PORK - UNII:AVT680JB39) INSULIN PORK 8 [hp_X] in 1 mL INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 12 [hp_C] in 1 mL PORK LIVER (UNII: 6EC706HI7F) (PORK LIVER - UNII:6EC706HI7F) PORK LIVER 10 [hp_X] in 1 mL SUS SCROFA PANCREAS (UNII: 9Y3J3362RY) (SUS SCROFA PANCREAS - UNII:9Y3J3362RY) SUS SCROFA PANCREAS 10 [hp_X] in 1 mL PHLORIZIN (UNII: CU9S17279X) (PHLORIZIN - UNII:CU9S17279X) PHLORIZIN 16 [hp_X] in 1 mL ALLOXAN (UNII: 6SW5YHA5NG) (ALLOXAN - UNII:6SW5YHA5NG) ALLOXAN 6 [hp_C] in 1 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 6 [hp_C] in 1 mL HUMAN BREAST TUMOR CELL (UNII: C62OO7VD9K) (HUMAN BREAST TUMOR CELL - UNII:C62OO7VD9K) HUMAN BREAST TUMOR CELL 6 [hp_C] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 6 [hp_C] in 1 mL MOMORDICA BALSAMINA IMMATURE FRUIT (UNII: WUW1665V10) (MOMORDICA BALSAMINA IMMATURE FRUIT - UNII:WUW1665V10) MOMORDICA BALSAMINA IMMATURE FRUIT 6 [hp_C] in 1 mL PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 6 [hp_C] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 6 [hp_C] in 1 mL SYZYGIUM CUMINI SEED (UNII: 820LSF646I) (SYZYGIUM CUMINI SEED - UNII:820LSF646I) SYZYGIUM CUMINI SEED 6 [hp_C] in 1 mL SALMONELLA ENTERICA SUBSP. ENTERICA SEROVAR ENTERITIDIS (UNII: Y3V16D4PV4) (SALMONELLA ENTERICA ENTERICA SEROVAR ENTERITIDIS - UNII:Y3V16D4PV4) SALMONELLA ENTERICA SUBSP. ENTERICA SEROVAR ENTERITIDIS 30 [hp_C] in 1 mL GYMNEMA SYLVESTRE LEAF (UNII: 2ZK6ZS8392) (GYMNEMA SYLVESTRE LEAF - UNII:2ZK6ZS8392) GYMNEMA SYLVESTRE LEAF 30 [hp_C] in 1 mL TRIGONELLA FOENUM-GRAECUM WHOLE (UNII: 5610HF69OB) (TRIGONELLA FOENUM-GRAECUM WHOLE - UNII:5610HF69OB) TRIGONELLA FOENUM-GRAECUM WHOLE 32 [hp_C] in 1 mL BARIUM OXALOSUCCINATE (UNII: L7A49804ZQ) (BARIUM CATION - UNII:V645272HLN) BARIUM OXALOSUCCINATE 32 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43742-2064-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 12/07/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/07/2022 Labeler - Deseret Biologicals, Inc. (940741853) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43742-2064) , api manufacture(43742-2064) , label(43742-2064) , pack(43742-2064)