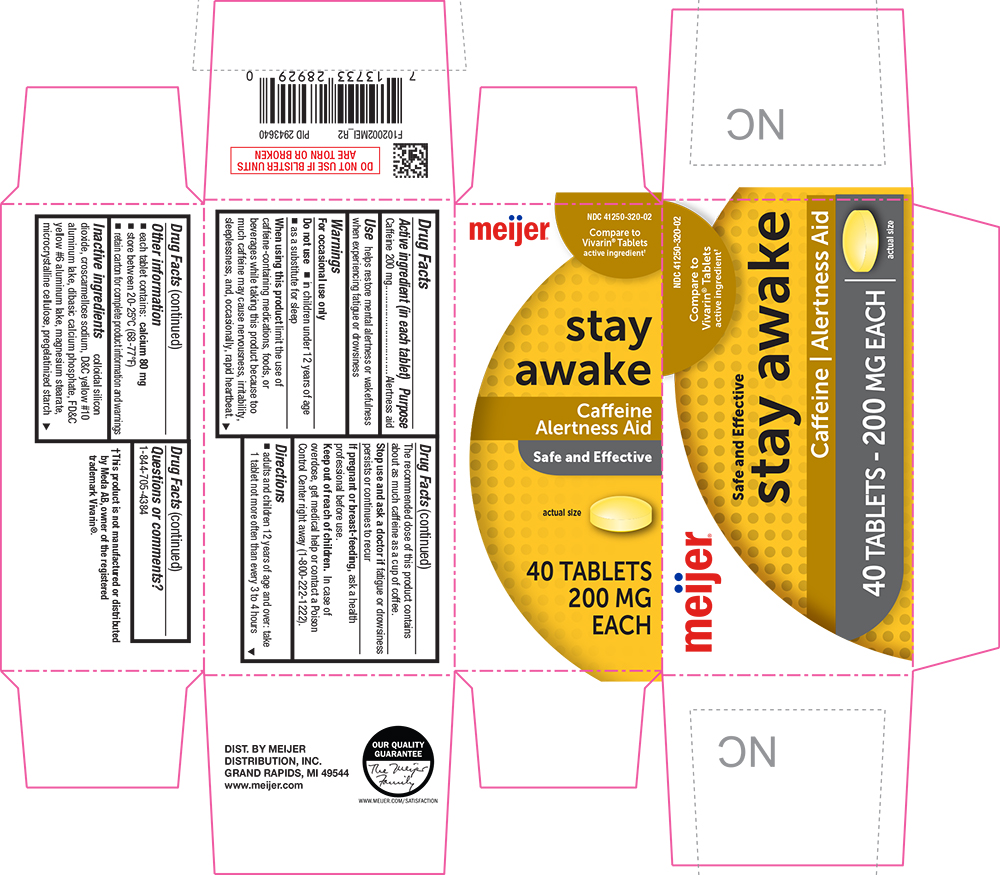
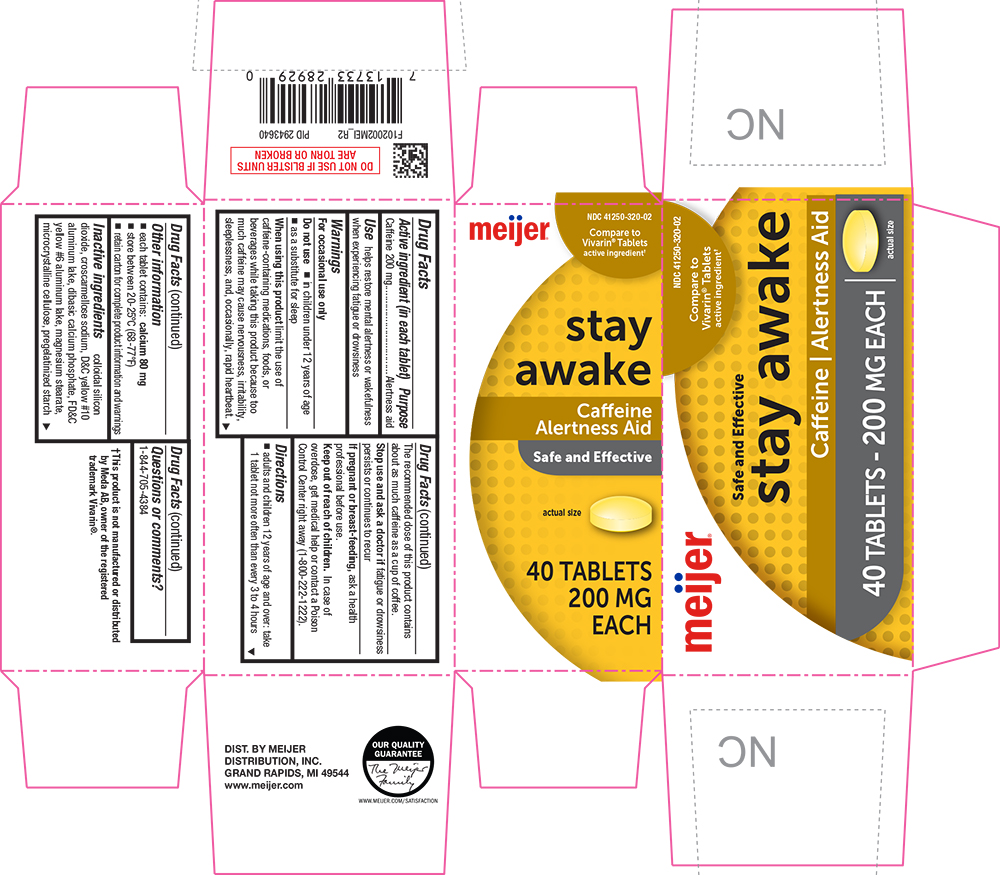
Label: STAY AWAKE- caffeine tablet
- NDC Code(s): 41250-320-02
- Packager: Meijer
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
- Use
-
Warnings
When using this product limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat. The recommended dose of this product contains about as much caffeine as a cup of coffee.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STAY AWAKE
caffeine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41250-320 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) ALUMINUM OXIDE (UNII: LMI26O6933) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) Product Characteristics Color yellow Score no score Shape ROUND Size 11mm Flavor Imprint Code LL64;57344 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41250-320-02 5 in 1 CARTON 08/01/2011 07/31/2025 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M011 08/01/2011 07/31/2025 Labeler - Meijer (006959555)