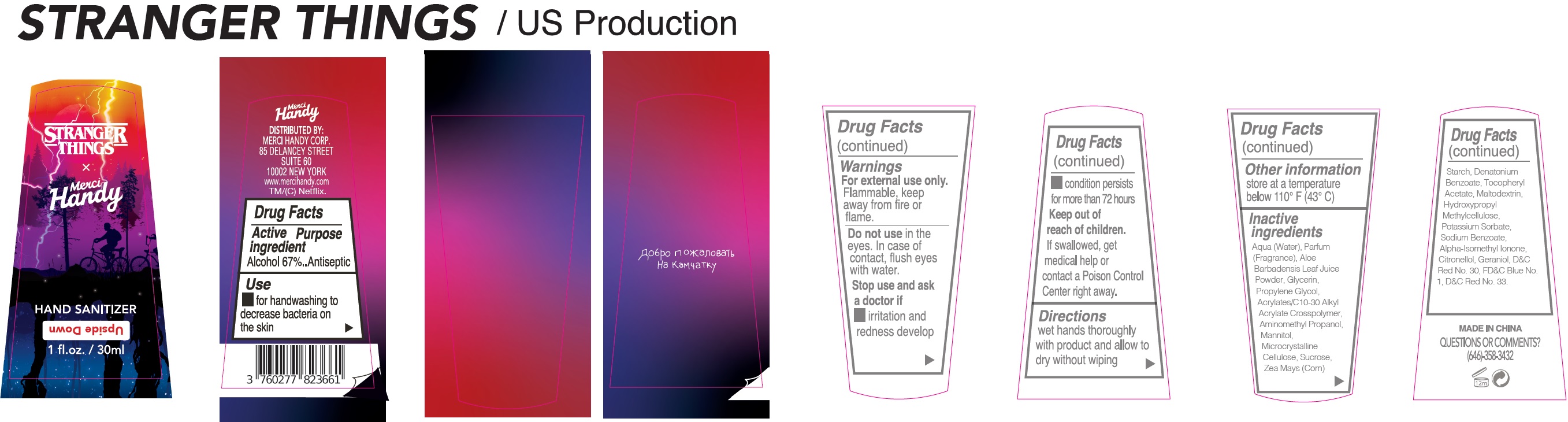
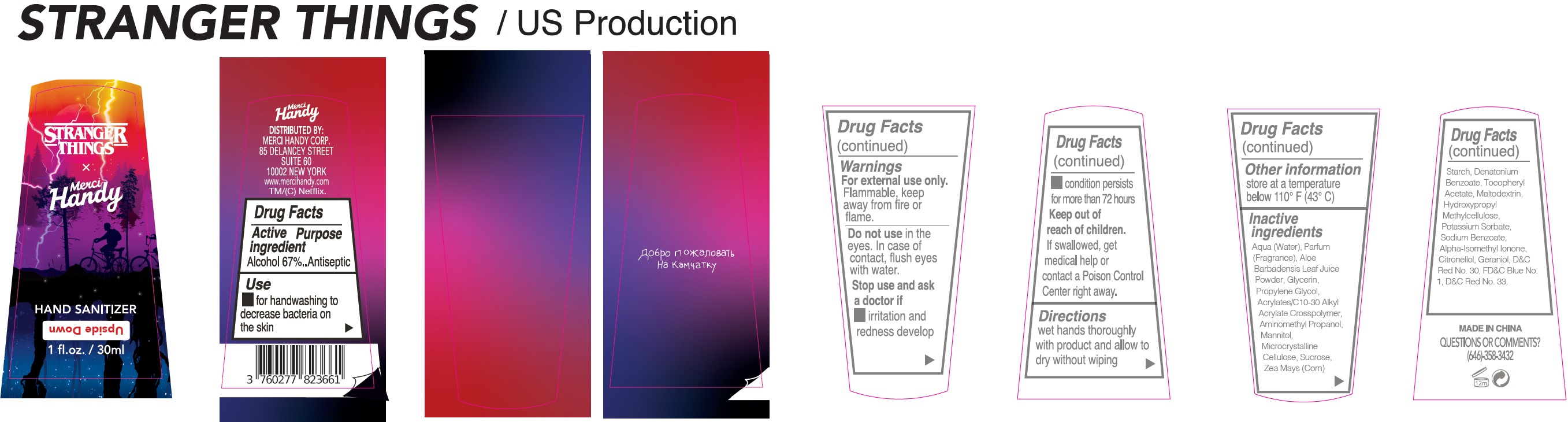
Label: UPSIDE DOWN COLLECTOR FANNY PACK- alcohol kit
- NDC Code(s): 72866-034-01, 72866-035-01
- Packager: MERCI HANDY CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aqua (Water), Parfum (Fragrance), Aloe Barbadensis Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Zea Mays (Corn) Starch, Denatonium Benzoate, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, Alpha-Isomethyl Ionone, Citronellol, Geraniol, CI 73360 (Red 30 Lake), CI 42090 (Blue 1), CI 17200 (Red 33).
- Package Labeling:72866-034-01
- Package Labeling:72866-035-01
-
INGREDIENTS AND APPEARANCE
UPSIDE DOWN COLLECTOR FANNY PACK
alcohol kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72866-034 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-034-01 1 in 1 POUCH 09/20/2021 12/31/2026 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 30 mL Part 1 of 1 HAND SANITIZER UPSIDE DOWN
alcohol gelProduct Information Item Code (Source) NDC:72866-035 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 67 mL in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) CORN (UNII: 0N8672707O) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) GERANIOL (UNII: L837108USY) D&C RED NO. 30 (UNII: 2S42T2808B) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-035-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/20/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/20/2021 12/31/2026 Labeler - MERCI HANDY CORPORATION (118006306)