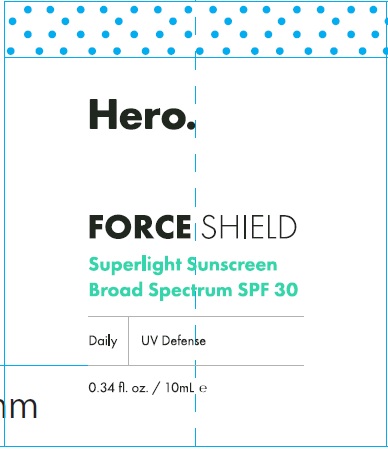
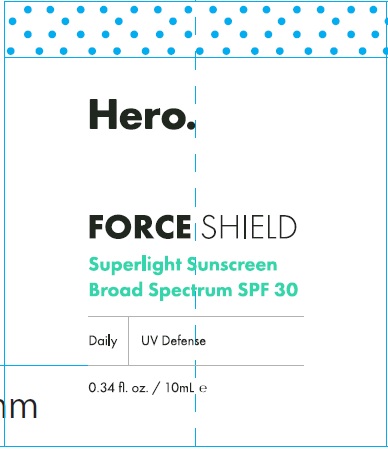
Label: HERO FORCE SHIELD SPF 30- zinc oxide cream
- NDC Code(s): 73381-506-10
- Packager: Hero Cosmetics, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 2, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m., Wear long-sleeved shirts, pants, hats and sunglasses.
- Children under 6 months: Ask a doctor.
- Other information
-
Inactive Ingredients
Water, Caprylic/Capric Triglyceride, C13-15 Alkane, Propanediol, C15-19 Alkane, Bis-Diglyceryl Polyacyladipate-2, Butyloctyl Salicylate, Ectoin, Alteromonas (Abyssine) Ferment Extract, Harungana Madagascariensis (Madagascar) Extract, Ipomoea Batatas (Purple Sweet Potato) Root Extract, Rubus Idaeus (Raspberry) Seed Oil, Camellia Sinensis (Green Tea) Leaf Extract, Corallina Officinalis (Red Algae) Extract, Curcuma Longa (Turmeric) Leaf Extract, Melia Azadirachta (Neem) Flower Extract, Melia Azadirachta (Neem) Leaf Extract, Moringa Oleifera Seed Oil, Ocimum Sanctum (Holy Basil) Leaf Extract, Oryza Sativa (Rice) Hull Extract, Solanum Melongena (Eggplant) Fruit Extract, Coccinia Indica (Ivy Gourd) Fruit Extract, Tocopherol (Vitamin E), Bisabolol, Amber Powder, Coco-Glucoside, Glucose, Glycerin, Xanthan Gum, 1,2-Hexanediol, Caprylhydroxamic Acid, Arachidyl Alcohol, Arachidyl Glucoside, Hydroxyacetophenone, Polyacrylate Crosspolymer-6, Behenyl Alcohol, Cetearyl Alcohol, Isostearic Acid, Lecithin, Polyglycerin-3, Polyglyceryl-3 Lactate/Laurate, Polyglyceryl-3 Polyricinoleate, Polyhydroxystearic Acid, Silica, t-Butyl Alcohol, Sodium Benzoate, Sodium Citrate, Sodium Dilauramidoglutamide Lysine, Sodium Phytate, Citric Acid, Inositol, Lactobacillus Ferment Lysate, Saccharomyces Lysate, Butylene Glycol, Caprylyl Glycol.
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
HERO FORCE SHIELD SPF 30
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73381-506 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 175.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) C13-15 ALKANE (UNII: 114P5I43UJ) PROPANEDIOL (UNII: 5965N8W85T) C15-19 ALKANE (UNII: CI87N1IM01) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ECTOINE (UNII: 7GXZ3858RY) ALTEROMONAS MACLEODII POLYSACCHARIDES (UNII: AP6XG2GR8Z) SWEET POTATO (UNII: M9WGG9Z9GK) RASPBERRY SEED OIL (UNII: 9S8867952A) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CORALLINA OFFICINALIS (UNII: 4004498D06) CURCUMA LONGA LEAF (UNII: H2HC4RY52C) AZADIRACHTA INDICA FLOWER (UNII: 3TE8A92UPM) AZADIRACHTA INDICA LEAF (UNII: HKY915780T) MORINGA OLEIFERA SEED OIL (UNII: REM6A5QMC0) HOLY BASIL LEAF (UNII: SCJ765569P) RICE BRAN (UNII: R60QEP13IC) EGGPLANT (UNII: W5K7RAS4VK) COCCINIA GRANDIS FRUIT (UNII: VLJ6WOT3K5) TOCOPHEROL (UNII: R0ZB2556P8) LEVOMENOL (UNII: 24WE03BX2T) AMBER (UNII: 70J9Z0J26P) COCO GLUCOSIDE (UNII: ICS790225B) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) DOCOSANOL (UNII: 9G1OE216XY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) POLYGLYCERYL-3 LAURATE (UNII: Y9ZSR39D0E) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) PHYTATE SODIUM (UNII: 88496G1ERL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INOSITOL (UNII: 4L6452S749) SACCHAROMYCES LYSATE (UNII: R85W246Z1C) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73381-506-10 10 mL in 1 TUBE; Type 0: Not a Combination Product 06/01/2021 10/24/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/01/2021 10/24/2024 Labeler - Hero Cosmetics, Inc. (053668306) Establishment Name Address ID/FEI Business Operations Innovation Labs, Inc. 117109069 manufacture(73381-506)