Label: DOC JOHNSON PROLONGING GINSENG- benzocaine cream
- NDC Code(s): 69503-003-01
- Packager: Health Devices Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
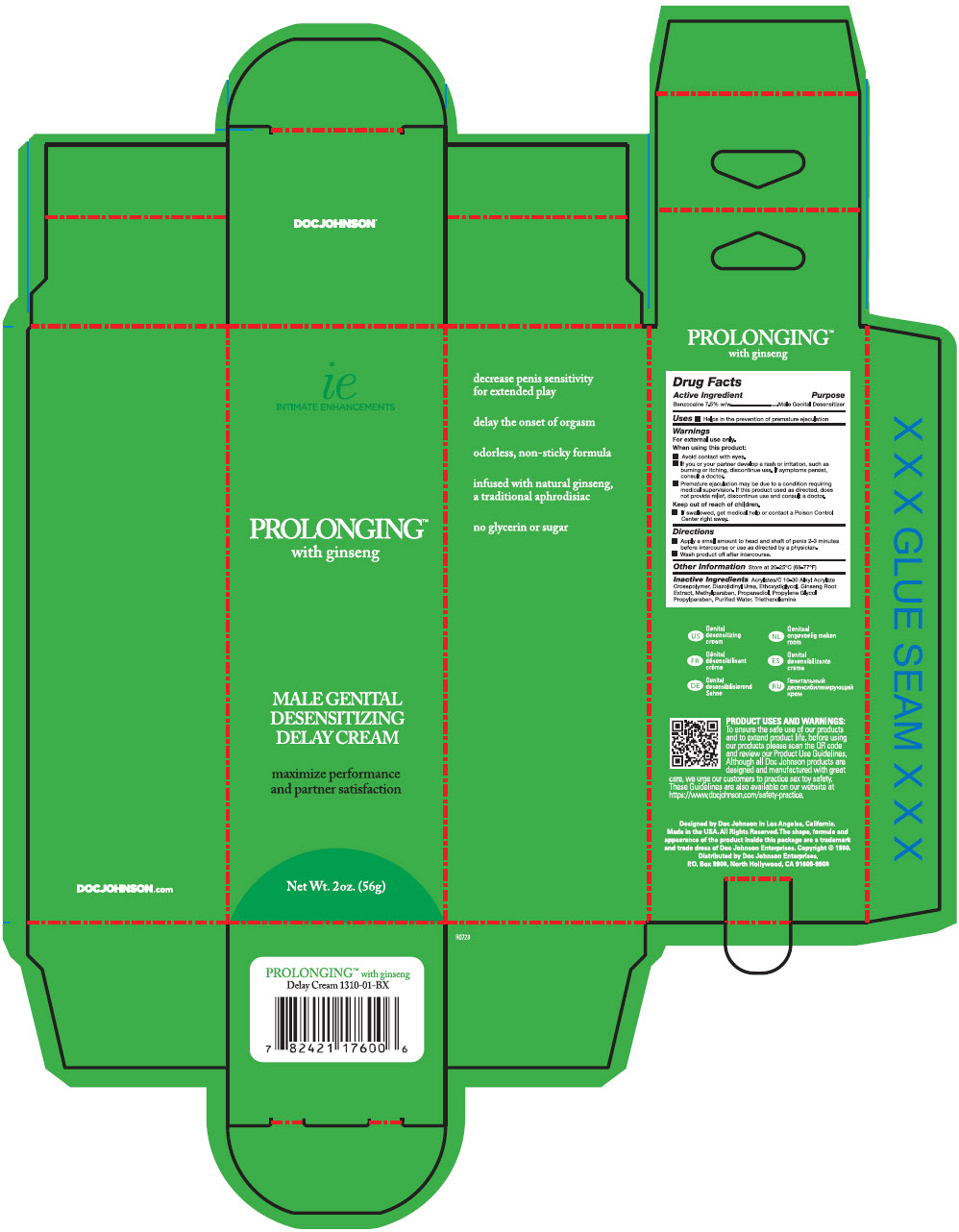
- Active Ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- Avoid contact with eyes
- If you or your partner develop a rash or irritation, such as burning or itching, discontinue use. If symptoms persist, consult a doctor.
- Premature ejaculation may be due to a condition requiring medical supervision. If this product used as directed, does not provide relief, discontinue use and consult a doctor.
- Directions
- Other Information
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 56 g Tube Carton
-
INGREDIENTS AND APPEARANCE
DOC JOHNSON PROLONGING GINSENG
benzocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69503-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 7.5 g in 100 g Inactive Ingredients Ingredient Name Strength CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) 0.3 g in 100 g DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) 0.3 g in 100 g DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) 15 g in 100 g METHYLPARABEN (UNII: A2I8C7HI9T) 0.11 g in 100 g ASIAN GINSENG (UNII: CUQ3A77YXI) 0.1 g in 100 g PROPANEDIOL (UNII: 5965N8W85T) 10 g in 100 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 0.56 g in 100 g PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.03 g in 100 g WATER (UNII: 059QF0KO0R) 65.8 g in 100 g TROLAMINE (UNII: 9O3K93S3TK) 0.3 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69503-003-01 56 g in 1 TUBE; Type 0: Not a Combination Product 05/23/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 05/23/2023 Labeler - Health Devices Corporation (085927861)