Label: GLEN PE- phenylephrine hydrochloride and pyrilamine maleate syrup
-
Contains inactivated NDC Code(s)
NDC Code(s): 70147-224-16 - Packager: Glendale Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 9, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
- nasal congestion
- temporarily restores freer breathing through the nose
- reduces swelling of nasal passages
-
Warnings
Do not exceed recommended dosage.
Do not use this product
- to sedate a child or make a child sleepy
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are
- taking any other nasal decongestant or stimulant
- taking sedatives or tranquilizers
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
- Directions
- Other information
- Inactive ingredients
- Questions? Comments?
- SPL UNCLASSIFIED SECTION
-
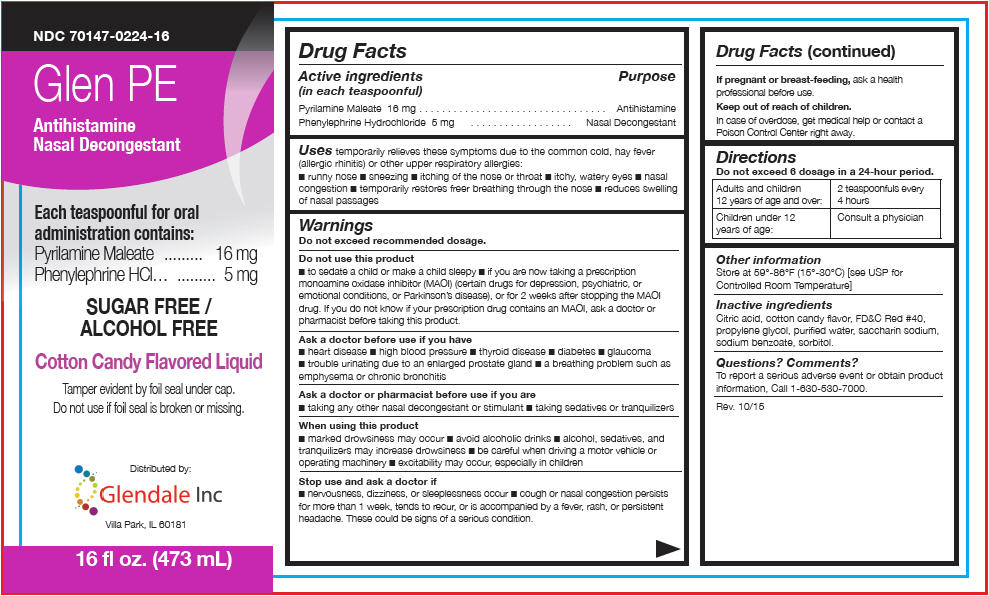
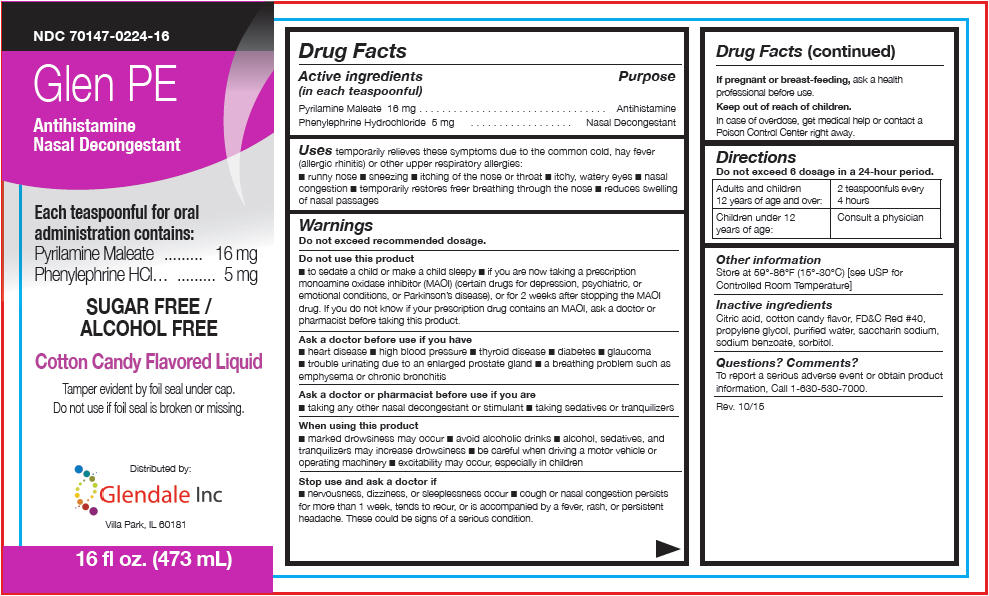
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
NDC 70147-0224-16
Glen PE
Antihistamine
Nasal DecongestantEach teaspoonful for oral
administration contains:
Pyrilamine Maleate 16 mg
Phenylephrine HCl 5 mgSUGAR FREE /
ALCOHOL FREECotton Candy Flavored Liquid
Tamper evident by foil seal under cap.
Do not use if foil seal is broken or missing.Distributed by:
Glendale Inc
Villa Park, IL 6018116 fl oz. (473 mL)
-
INGREDIENTS AND APPEARANCE
GLEN PE
phenylephrine hydrochloride and pyrilamine maleate syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70147-224 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 15 mg in 5 mL PYRILAMINE MALEATE (UNII: R35D29L3ZA) (PYRILAMINE - UNII:HPE317O9TL) PYRILAMINE MALEATE 16 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) FD&C red no. 40 (UNII: WZB9127XOA) Product Characteristics Color Score Shape Size Flavor COTTON CANDY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70147-224-16 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 12/05/2015 Labeler - Glendale Inc (079987961)