Label: DR. PELO SCALP TONIC SHEET- salicylic acid liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 81702-205-01 - Packager: JRJ.,INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
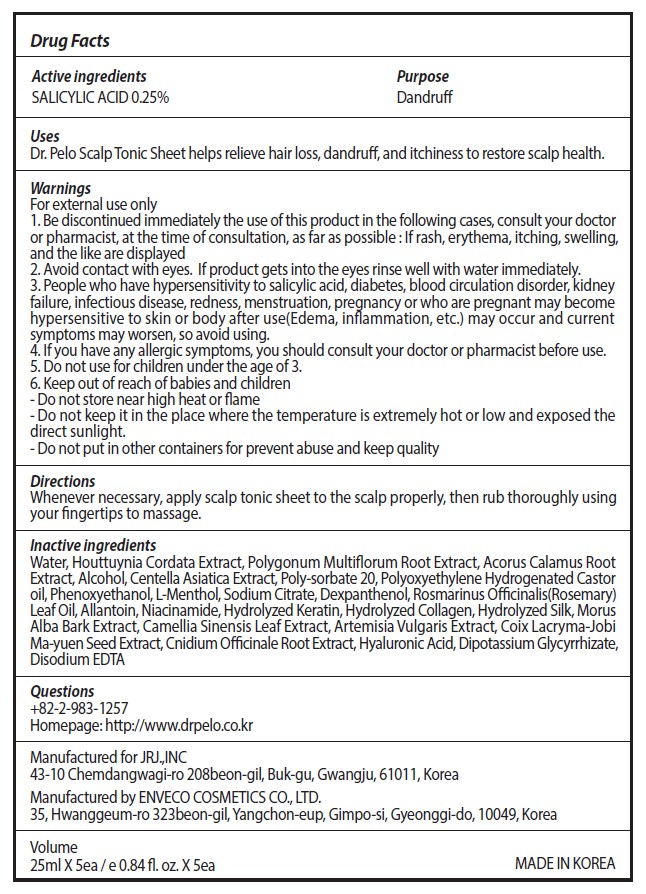
Drug Label Information
Updated July 9, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Stop use
1. Be discontinued immediately the use of this product in the following cases, consult your doctor or pharmacist, at the time of consultation, as far as possible : If rash, erythema, itching, swelling, and the like are displayed
2. Avoid contact with eyes. If product gets into the eyes rinse well with water immediately.
3. People who have hypersensitivity to salicylic acid, diabetes, blood circulation disorder, kidney failure, infectious disease, redness, menstruation, pregnancy or who are pregnant may become hypersensitive to skin or body after use(Edema, inflammation, etc.) may occur and current symptoms may worsen, so avoid using.
4. If you have any allergic symptoms, you should consult your doctor or pharmacist before use.
- Do not use
- Keep out of reach of children
- Directions
-
Inactive ingredients
Water, Houttuynia Cordata Extract, Polygonum Multiflorum Root Extract, Acorus Calamus Root
Extract, Alcohol, Centella Asiatica Extract, Poly-sorbate 20, Polyoxyethylene Hydrogenated Castor
oil, Phenoxyethanol, L-Menthol, Sodium Citrate, Dexpanthenol, Rosmarinus
Officinalis(Rosemary) Leaf Oil, Allantoin, Niacinamide, Hydrolyzed Keratin, Hydrolyzed Collagen,
Hydrolyzed Silk, Morus Alba Bark Extract, Camellia Sinensis Leaf Extract, Artemisia Vulgaris Extract,
Coix Lacryma-Jobi Ma-yuen Seed Extract, Cnidium Officinale Root Extract, Hyaluronic Acid,
Dipotassium Glycyrrhizate, Disodium EDTA - Other information
- Package Label
-
INGREDIENTS AND APPEARANCE
DR. PELO SCALP TONIC SHEET
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81702-205 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.25 mg in 100 mL Inactive Ingredients Ingredient Name Strength CENTELLA ASIATICA WHOLE (UNII: 7M867G6T1U) LEVOMENTHOL (UNII: BZ1R15MTK7) ROSEMARY OIL (UNII: 8LGU7VM393) GREEN TEA LEAF (UNII: W2ZU1RY8B0) COIX LACRYMA-JOBI VAR. MA-YUEN SEED (UNII: 8DW238I7ZI) MORUS ALBA BARK (UNII: 7O71A48NDP) WATER (UNII: 059QF0KO0R) ACORUS CALAMUS ROOT (UNII: XY1K7KIQ0F) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) REYNOUTRIA MULTIFLORA ROOT (UNII: AUZ3VD75MC) ALCOHOL (UNII: 3K9958V90M) POLYSORBATE 20 (UNII: 7T1F30V5YH) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) DEXPANTHENOL (UNII: 1O6C93RI7Z) ALLANTOIN (UNII: 344S277G0Z) NIACINAMIDE (UNII: 25X51I8RD4) ARTEMISIA VULGARIS ROOT (UNII: 32MP823R8S) CNIDIUM OFFICINALE ROOT (UNII: 8S3OZD358J) HYALURONIC ACID (UNII: S270N0TRQY) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81702-205-01 25 mL in 1 POUCH; Type 0: Not a Combination Product 03/22/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 03/22/2021 Labeler - JRJ.,INC (695134315) Registrant - JRJ.,INC (695134315) Establishment Name Address ID/FEI Business Operations JRJ.,INC 695134315 manufacture(81702-205)