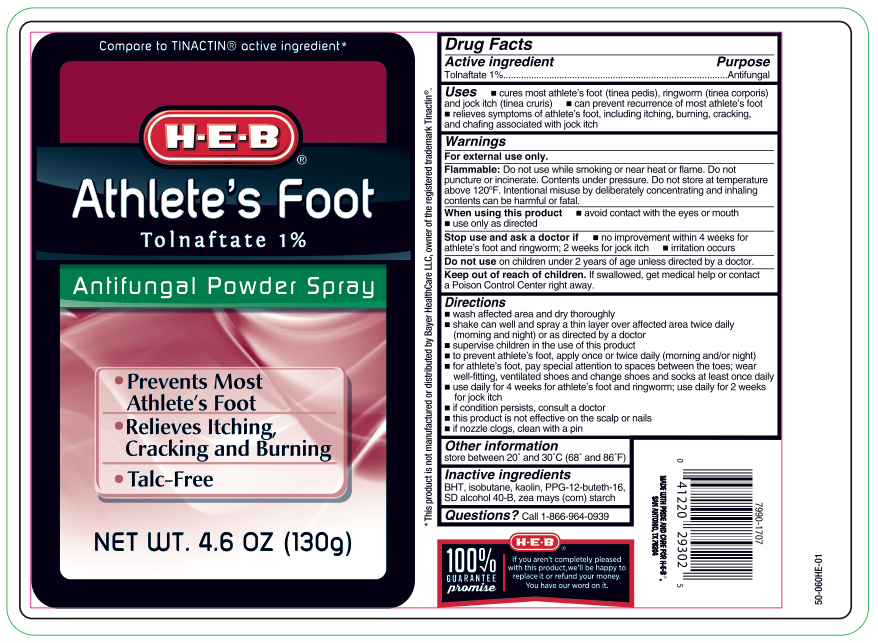
Label: TOLNAFATE- tolnaftate powder spray aerosol, spray
- NDC Code(s): 37808-530-46
- Packager: HEB
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
WARNINGS
For external use only.
Flammable:
Do not use while smoking or near heat or flame. Do not puncture or incinerate. Contents under pressure. Do not store at temperature above 120ºF
-
Directions
- wash affected area and dry thoroughly
- shake can well and spray a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- for athlete's foot: pay special attention to spaces between the toes, wear well-fitting, ventilated shoes and change shoes and socks at least once daily
- use daily for 4 weeks; if conditions persist, consult a doctorto prevent athlete's foot, apply once or twice daily (morning and/or night)in case of clogging, clear nozzle under running water
- Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TOLNAFATE
tolnaftate powder spray aerosol, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37808-530 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1.3 g in 130 g Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ISOBUTANE (UNII: BXR49TP611) KAOLIN (UNII: 24H4NWX5CO) PPG-12-BUTETH-16 (UNII: 58CG7042J1) ALCOHOL (UNII: 3K9958V90M) ZEA MAYS SUBSP. MAYS WHOLE (UNII: 1G5HNE09V8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37808-530-46 130 g in 1 CAN; Type 0: Not a Combination Product 12/22/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 12/22/2017 Labeler - HEB (007924756)