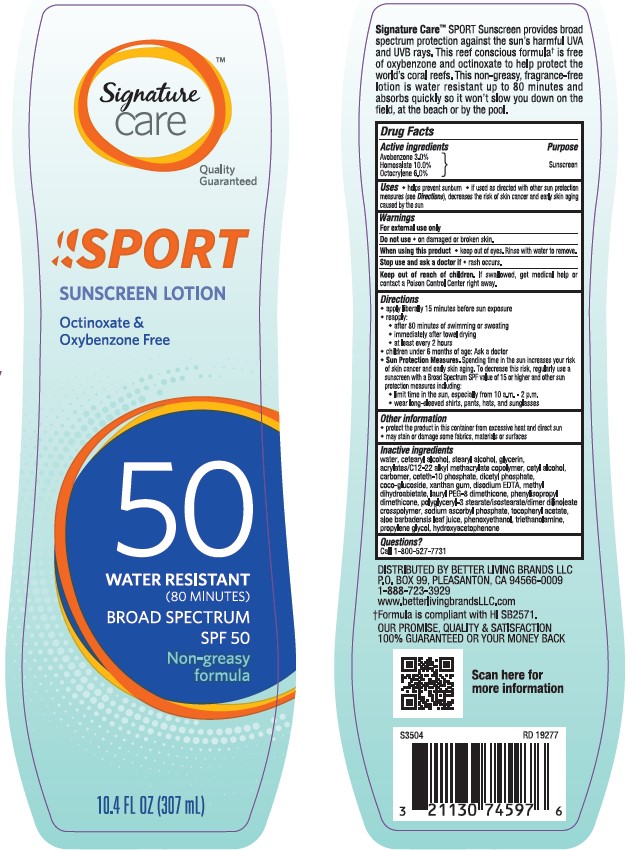
Label: SAFEWAY SIGNATURE CARE SPORT SUNSCREEN SPF 50- avobenzone, homosalate, octocrylene lotion
- NDC Code(s): 21130-731-40
- Packager: Safeway, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
• apply liberally 15 minutes before sun exposure
•reapply:
• after 80 minutes of swimming or sweating
• immediately after towel drying
• at least every 2 hours
• children under 6 months of age: Ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. - 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
- Other information
-
Inactive ingredients
water, cetearyl alcohol, stearyl alcohol, glycerin, acrylates/C12-22 alkyl methacrylate copolymer, cetyl alcohol, carbomer, ceteth-10 phosphate, dicetyl phosphate, coco-glucoside, xanthan gum, disodium EDTA, methyl dihydroabietate, lauryl PEG-8 dimethicone, phenylisopropyl dimethicone, polyglyceryl-3 stearate/isostearate/dimer dilinoleate crosspolymer, sodium ascorbyl phosphate, tocopheryl acetate, aloe barbadensis leaf juice, phenoxyethanol, triethanolamine, propylene glycol, hydroxyacetophenone
- Label
-
INGREDIENTS AND APPEARANCE
SAFEWAY SIGNATURE CARE SPORT SUNSCREEN SPF 50
avobenzone, homosalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21130-731 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) CETETH-10 PHOSPHATE (UNII: 4E05O5N49G) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) EDETATE DISODIUM (UNII: 7FLD91C86K) METHYL DIHYDROABIETATE (UNII: 7666FJ0J9F) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TROLAMINE (UNII: 9O3K93S3TK) ALOE VERA LEAF (UNII: ZY81Z83H0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) LAURYL PEG/PPG-18/18 METHICONE (UNII: ZJ5S27D9NX) COCO GLUCOSIDE (UNII: ICS790225B) BUTYL ACRYLATE/C16-C20 ALKYL METHACRYLATE/METHACRYLIC ACID/METHYL METHACRYLATE COPOLYMER (UNII: 7K68DGG29P) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CETYL ALCOHOL (UNII: 936JST6JCN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21130-731-40 307 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/14/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/14/2011 Labeler - Safeway, Inc. (009137209)