Label: CERAVE DEVELOPED WITH DERMATOLOGISTS ITCH RELIEF MOISTURIZING- pramoxine hydrochloride cream
- NDC Code(s): 49967-512-01, 49967-512-02, 49967-512-03
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
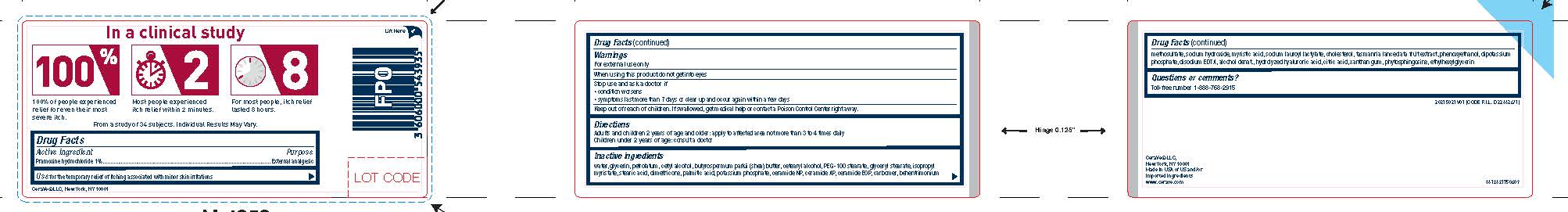
- Active ingredient
- Purpose
- Use
- Warnings
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Inactive ingredients
water, glycerin, petrolatum, cetyl alcohol, butyrospermum parkii (shea) butter, cetearyl alcohol, PEG-100 stearate, glyceryl stearate, isopropyl myristate, stearic acid, dimethicone, palmitic acid, potassium phosphate, ceramide NP, ceramide AP, ceramide EOP, carbomer, behentrimonium methosulfate, sodium hydroxide, myristic acid, sodium lauroyl lactylate, cholesterol, tasmannia lanceolata fruit extract, phenoxyethanol, dipotassium phosphate, disodium EDTA, alcohol denat., hydrolyzed hyaluronic acid, citric acid, xanthan gum, hytosphingosine, ethylhexylglycerin - Questions or comments?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CERAVE DEVELOPED WITH DERMATOLOGISTS ITCH RELIEF MOISTURIZING
pramoxine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-512 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PETROLATUM (UNII: 4T6H12BN9U) CETYL ALCOHOL (UNII: 936JST6JCN) STEARIC ACID (UNII: 4ELV7Z65AP) SHEA BUTTER (UNII: K49155WL9Y) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) DIMETHICONE (UNII: 92RU3N3Y1O) PALMITIC ACID (UNII: 2V16EO95H1) MYRISTIC ACID (UNII: 0I3V7S25AW) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) PHENOXYETHANOL (UNII: HIE492ZZ3T) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM HYDROXIDE (UNII: 55X04QC32I) ALCOHOL (UNII: 3K9958V90M) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) EDETATE DISODIUM (UNII: 7FLD91C86K) POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) TASMANNIA LANCEOLATA FRUIT (UNII: PNT2HDL13Q) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) CHOLESTEROL (UNII: 97C5T2UQ7J) HYALURONIC ACID (UNII: S270N0TRQY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CARBOMER HOMOPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: F68VH75CJC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-512-01 340 g in 1 JAR; Type 0: Not a Combination Product 09/18/2017 2 NDC:49967-512-02 453.6 g in 1 JAR; Type 0: Not a Combination Product 09/18/2017 3 NDC:49967-512-03 538.6 g in 1 JAR; Type 0: Not a Combination Product 09/18/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/18/2017 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations Accupac, Inc. 071609663 MANUFACTURE(49967-512)