Label: PROPOLIS NASAL PLUS- plus nasal liquid
PROPOLIS NASAL- nasal liquid
SUNSCREEN SPF50- non nano zinc oxide cream
SUNSCREEN FOR FACE SPF50- non nano zinc oxide cream
AFTERSUN- non nano zinc oxide cream
DIAPER RASH CREAM- non nano zinc oxide cream
SUNSCREEN FOR KIDS SPF50- non nano zinc oxide cream
S.O.S CREAM INTENSIVE MOISTURIZING SKIN PROTECTANT- non nano zinc oxide cream
ACNE CONTROL SERUM- salicylic acid liquid
-
NDC Code(s):
82123-004-80,
82123-004-81,
82123-005-50,
82123-005-51, view more82123-007-15, 82123-007-16, 82123-008-30, 82123-008-31, 82123-009-30, 82123-009-31, 82123-010-30, 82123-010-31, 82123-011-80, 82123-011-81, 82123-012-80, 82123-012-81, 82123-013-40, 82123-013-41
- Packager: SBS BILIMSEL BIO COZUMLER SANAYI VE TICARET ANONIM SIRKETI PINAR SUBESI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
-
WHEN USING
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive Ingredients
- Acne Control Serum
- Sunscreen for kids SPF50
- Sunscreen for face SPF50
- Aftersun
- Diaper Rash Cream
- Propolis Nasal Plus
- Propolis Nasal
- SOS Cream
- Sunscreen SPF50
-
INGREDIENTS AND APPEARANCE
PROPOLIS NASAL PLUS
plus nasal liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82123-010 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.015 mg in 30 mL Inactive Ingredients Ingredient Name Strength EUCALYPTUS OIL (UNII: 2R04ONI662) WATER (UNII: 059QF0KO0R) SEA SALT (UNII: 87GE52P74G) PROPOLIS WAX (UNII: 6Y8XYV2NOF) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82123-010-31 1 in 1 BOX 10/03/2023 1 NDC:82123-010-30 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/03/2023 PROPOLIS NASAL
nasal liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82123-009 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.015 mg in 30 mL Inactive Ingredients Ingredient Name Strength EUCALYPTUS OIL (UNII: 2R04ONI662) SEA SALT (UNII: 87GE52P74G) GLYCERIN (UNII: PDC6A3C0OX) PROPOLIS WAX (UNII: 6Y8XYV2NOF) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82123-009-31 1 in 1 BOX 10/02/2023 1 NDC:82123-009-30 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/02/2023 SUNSCREEN SPF50
non nano zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82123-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 12 mg in 80 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) JOJOBA OIL (UNII: 724GKU717M) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPOLIS WAX (UNII: 6Y8XYV2NOF) PANTHENOL (UNII: WV9CM0O67Z) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) MAGNESIUM SULFATE ANHYDROUS (UNII: ML30MJ2U7I) ALOE VERA LEAF (UNII: ZY81Z83H0X) ARGAN OIL (UNII: 4V59G5UW9X) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) GLYCERYL CITRATE (UNII: 4987GT719I) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82123-011-81 1 in 1 TUBE 06/28/2021 1 NDC:82123-011-80 80 mL in 1 TUBE; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/28/2021 SUNSCREEN FOR FACE SPF50
non nano zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82123-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6 mg in 40 mL Inactive Ingredients Ingredient Name Strength COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) ALOE VERA LEAF (UNII: ZY81Z83H0X) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) PANTHENOL (UNII: WV9CM0O67Z) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) MAGNESIUM SULFATE ANHYDROUS (UNII: ML30MJ2U7I) GLYCERYL CITRATE (UNII: 4987GT719I) PROPOLIS WAX (UNII: 6Y8XYV2NOF) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) ARGAN OIL (UNII: 4V59G5UW9X) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82123-013-41 1 in 1 TUBE 06/28/2021 1 NDC:82123-013-40 40 mL in 1 TUBE; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/28/2021 AFTERSUN
non nano zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82123-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1.6 mg in 80 mL Inactive Ingredients Ingredient Name Strength PROPOLIS WAX (UNII: 6Y8XYV2NOF) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) PHYSALIS ANGULATA (UNII: W4TKW9D5GG) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERYL CITRATE (UNII: 4987GT719I) WHITE WAX (UNII: 7G1J5DA97F) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) PANTHENOL (UNII: WV9CM0O67Z) WATER (UNII: 059QF0KO0R) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) SESAME OIL (UNII: QX10HYY4QV) XANTHAN GUM (UNII: TTV12P4NEE) ARGAN OIL (UNII: 4V59G5UW9X) MAGNESIUM SULFATE ANHYDROUS (UNII: ML30MJ2U7I) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82123-004-81 1 in 1 TUBE 06/28/2021 1 NDC:82123-004-80 80 mL in 1 TUBE; Type 0: Not a Combination Product 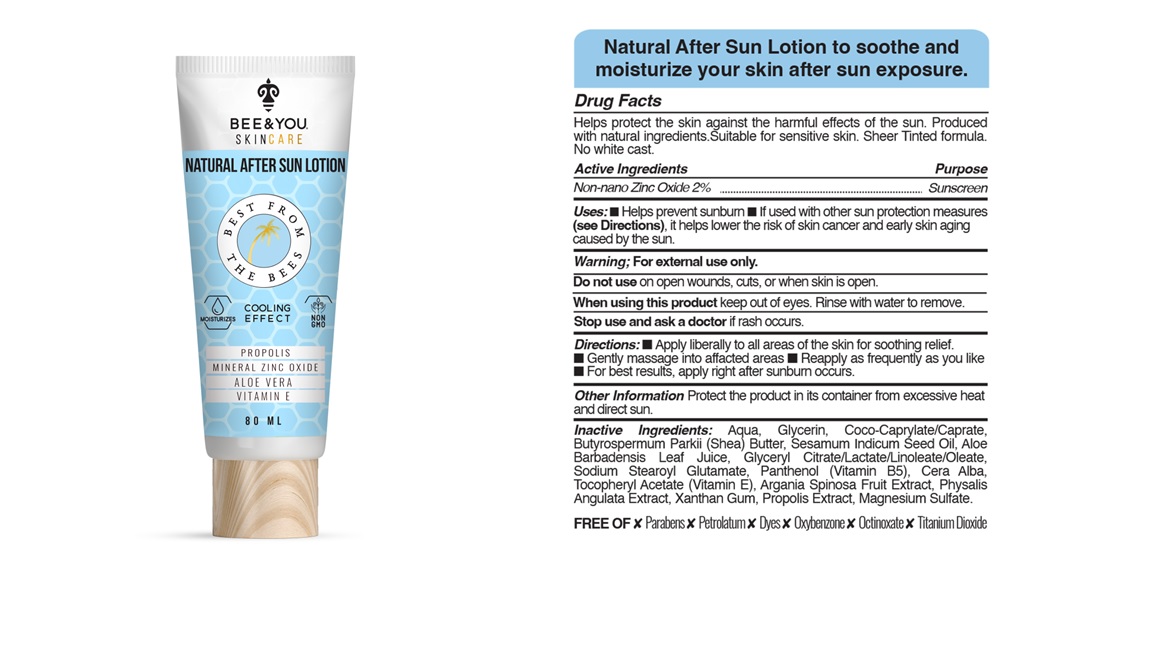
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/28/2021 DIAPER RASH CREAM
non nano zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82123-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 11 mg in 50 mg Inactive Ingredients Ingredient Name Strength BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) HYDROLYZED JOJOBA ESTERS (POTASSIUM SALTS) (UNII: CH428W5O62) WHITE WAX (UNII: 7G1J5DA97F) POLYGLYCERYL-3 OLEATE (UNII: XRQ165498B) PANTHENOL (UNII: WV9CM0O67Z) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CAPRYLIC/CAPRIC/LAURIC TRIGLYCERIDE (UNII: FJ1H6M2JG9) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) JOJOBA OIL (UNII: 724GKU717M) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) PALM OIL (UNII: 5QUO05548Z) PALM KERNEL OIL (UNII: B0S90M0233) COCONUT OIL (UNII: Q9L0O73W7L) CANOLA OIL (UNII: 331KBJ17RK) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82123-005-51 1 in 1 TUBE 06/28/2021 1 NDC:82123-005-50 50 mg in 1 TUBE; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/28/2021 SUNSCREEN FOR KIDS SPF50
non nano zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82123-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 17.6 mg in 80 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM SULFATE ANHYDROUS (UNII: ML30MJ2U7I) PALM KERNEL OIL (UNII: B0S90M0233) COCONUT OIL (UNII: Q9L0O73W7L) CANOLA OIL (UNII: 331KBJ17RK) WATER (UNII: 059QF0KO0R) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) ARGAN OIL (UNII: 4V59G5UW9X) GLYCERYL CITRATE (UNII: 4987GT719I) PROPOLIS WAX (UNII: 6Y8XYV2NOF) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PANTHENOL (UNII: WV9CM0O67Z) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) ALOE VERA LEAF (UNII: ZY81Z83H0X) PALM OIL (UNII: 5QUO05548Z) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82123-012-81 1 in 1 TUBE 06/28/2021 1 NDC:82123-012-80 80 mg in 1 TUBE; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/28/2021 S.O.S CREAM INTENSIVE MOISTURIZING SKIN PROTECTANT
non nano zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82123-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.6 mg in 15 mL Inactive Ingredients Ingredient Name Strength OLEA EUROPAEA FLOWER (UNII: 498M34P1VZ) WHITE WAX (UNII: 7G1J5DA97F) PANTHENOL (UNII: WV9CM0O67Z) HONEY (UNII: Y9H1V576FH) PROPOLIS WAX (UNII: 6Y8XYV2NOF) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) GLYCERIN (UNII: PDC6A3C0OX) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) WATER (UNII: 059QF0KO0R) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) ROYAL JELLY (UNII: L497I37F0C) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82123-007-16 1 in 1 BOX 11/11/2021 1 NDC:82123-007-15 15 mL in 1 CONTAINER; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/11/2021 ACNE CONTROL SERUM
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82123-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.15 mg in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) APIS MELLIFERA VENOM (UNII: 76013O881M) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) BEET (UNII: N487KM8COK) CAPRYLIC ACID (UNII: OBL58JN025) ROSA MULTIFLORA FRUIT (UNII: EZ5DSL4T27) HYALURONIC ACID (UNII: S270N0TRQY) ACTINIDIA POLYGAMA FRUIT (UNII: CJA97047JF) XYLITOL (UNII: VCQ006KQ1E) TEA TREE OIL (UNII: VIF565UC2G) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) PROPOLIS WAX (UNII: 6Y8XYV2NOF) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82123-008-31 1 in 1 BOX 10/11/2023 1 NDC:82123-008-30 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 09/29/2023 Labeler - SBS BILIMSEL BIO COZUMLER SANAYI VE TICARET ANONIM SIRKETI PINAR SUBESI (533166749) Registrant - SBS BILIMSEL BIO COZUMLER SANAYI VE TICARET ANONIM SIRKETI PINAR SUBESI (533166749) Establishment Name Address ID/FEI Business Operations SBS BILIMSEL BIO COZUMLER SANAYI VE TICARET ANONIM SIRKETI PINAR SUBESI 533166749 manufacture(82123-012, 82123-013, 82123-011, 82123-004, 82123-005, 82123-007, 82123-008, 82123-009, 82123-010)


















