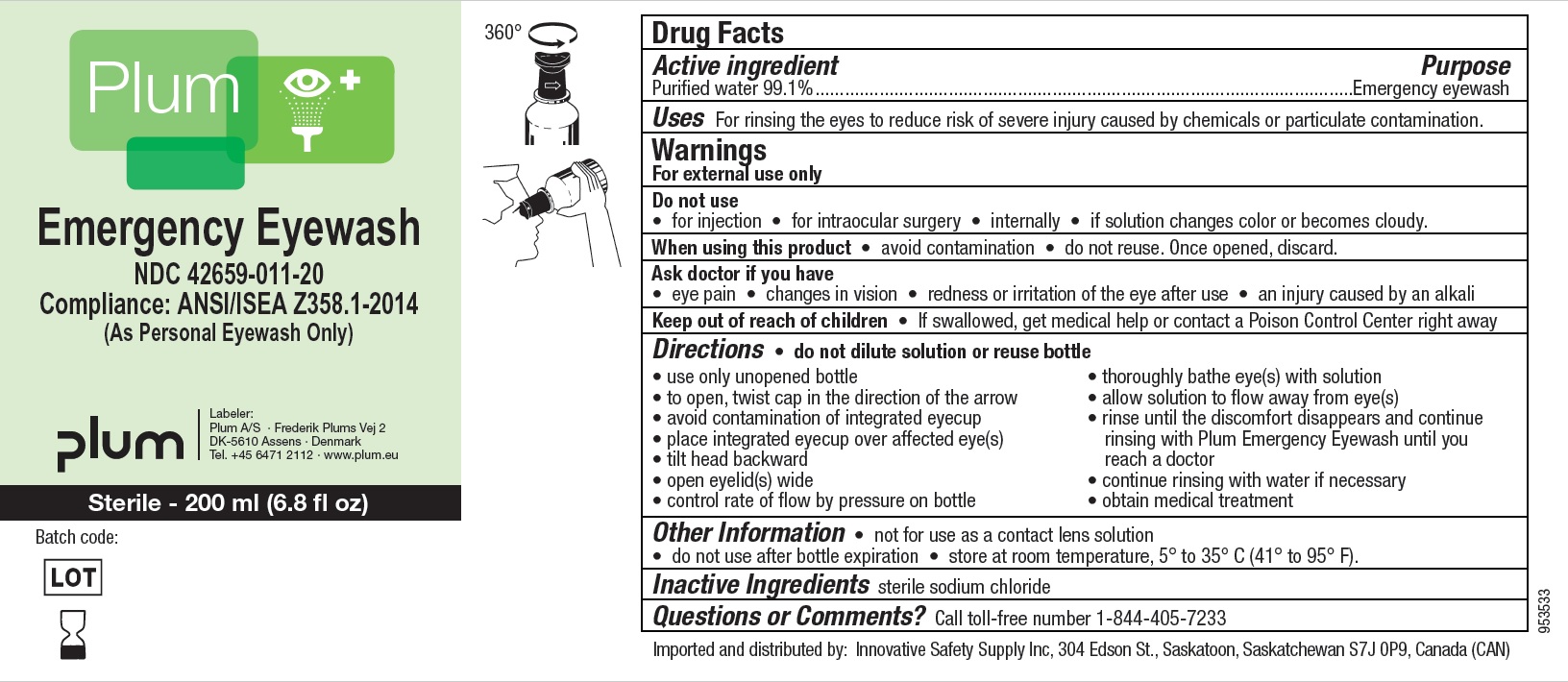
Label: PLUM EMERGENCY EYEWASH/EYEWASH DUO/EYEWASH SHOWER ISS USA- water liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 42659-011-20, 42659-011-21, 42659-011-22, 42659-011-23, view more42659-011-24, 42659-011-26, 42659-011-27 - Packager: Plum A/S
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 17, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Ask doctor if you have
- Keep out of reach of children
-
Directions
- do not dilute solution or reuse bottle
- use only unopened bottle
- to open, twist cap in the direction of the arrow
- avoid contamination of the integrated eyecup
- place integrated eyecup over affected eye(s)
- tilt head backward
- open eyelid(s) wide
- control rate of flow by pressure on bottle
- thoroughly bathe eye(s) with solution
- allow solution to flow away from eye(s)
- rinse until the bottle is empty and continue rinsing with Plum Emergency Eyewash until you reach a doctor
- continue rinsing with water if necessary
- obtain medical treatment.
- Other Information
- Inactive ingredients
- Questions or Comments
- Imported and distributed by
-
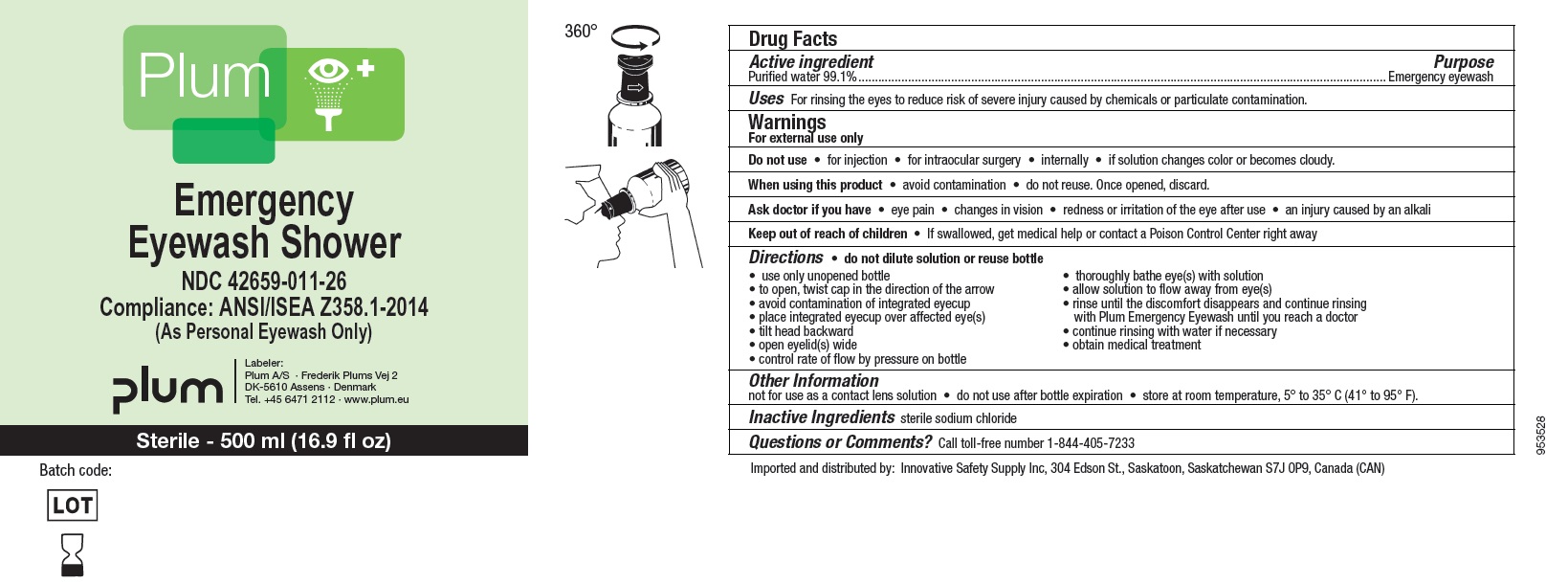
Principal Display Panel
NDC : 42659-011-20
Plum
Emergency Eyewash
Compliance: ANSI/ISEA Z358.1-2014
(As Personal Eyewash Only)
plum
Labeler: Plum A/S - Frederik plums Vej 2
DK - 5610 Assens - Denmark
Tel. +45 6471 2112
www.plum.eu
Sterile - 200 ml (6.8 fl oz)
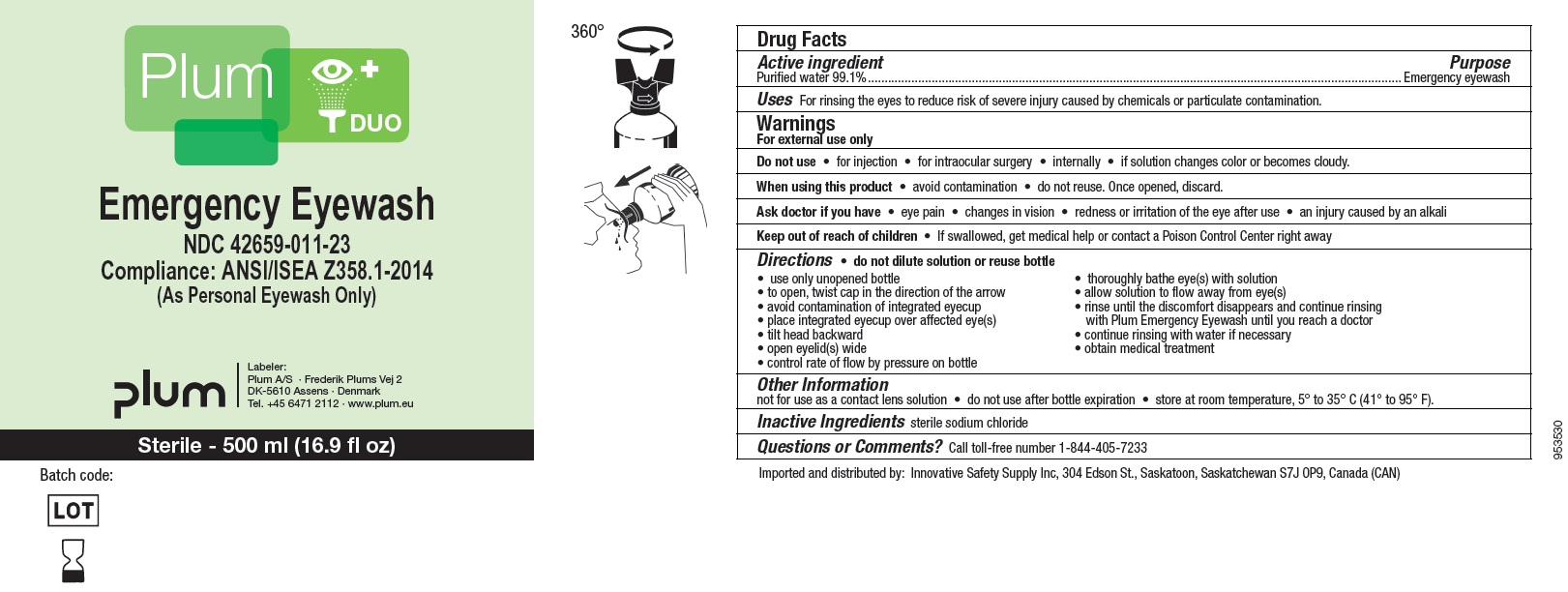
NDC : 42659-011-23
Plum DUO
Emergency Eyewash
Compliance: ANSI/ISEA Z358.1-2014
(As Personal Eyewash Only)
plum
Labeler: Plum A/S - Frederik plums Vej 2
DK - 5610 Assens - Denmark
Tel. +45 6471 2112
www.plum.eu
Sterile - 500 ml (16.9 fl oz)
NDC : 42659-011-26
Plum
Emergency Eyewash Shower
Compliance: ANSI/ISEA Z358.1-2014
(As Personal Eyewash Only)
plum
Labeler: Plum A/S - Frederik plums Vej 2
DK - 5610 Assens - Denmark
Tel. +45 6471 2112
www.plum.eu
Sterile - 500 ml (16.9 fl oz)
-
INGREDIENTS AND APPEARANCE
PLUM EMERGENCY EYEWASH/EYEWASH DUO/EYEWASH SHOWER ISS USA
water liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42659-011 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Water (UNII: 059QF0KO0R) (Water - UNII:059QF0KO0R) Water 99.1 mL in 100 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42659-011-20 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 2 NDC:42659-011-21 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 3 NDC:42659-011-22 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 4 NDC:42659-011-23 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 5 NDC:42659-011-24 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 6 NDC:42659-011-26 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 7 NDC:42659-011-27 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 06/17/2016 Labeler - Plum A/S (305299489) Establishment Name Address ID/FEI Business Operations Plum A/S 305299489 label(42659-011) Establishment Name Address ID/FEI Business Operations Holopack Verpackungstechnik GmbH 343390324 manufacture(42659-011)