Label: TROGARZO- ibalizumab injection, solution
- NDC Code(s): 62064-122-01, 62064-122-02
- Packager: Theratechnologies Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TROGARZO safely and effectively. See full prescribing information for TROGARZO.
TROGARZO® (ibalizumab-uiyk) injection, for intravenous use
Initial U.S. Approval: 2018INDICATIONS AND USAGE
TROGARZO, a CD4-directed post-attachment HIV-1 inhibitor, in combination with other antiretroviral(s), is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily treatment-experienced adults with multidrug resistant HIV-1 infection failing their current antiretroviral regimen. (1)
DOSAGE AND ADMINISTRATION
The recommended dosage regimen is a single loading dose of 2,000 mg followed by a maintenance dose of 800 mg every 2 weeks administered as a diluted intravenous infusion (IV infusion) or undiluted intravenous push (IV push). (2.1, 2.2, 2.3)
Duration of IV Infusion or IV Push
IV Infusion (Diluted) IV Push (Undiluted) Loading Dose 2,000 mg Over at least 30 minutes Over at least 90 seconds Maintenance Dose 800 mg Over at least 15 minutes Over at least 30 seconds DOSAGE FORMS AND STRENGTHS
Injection: 200 mg/1.33 mL (150 mg/mL) in a single-dose vial. (3)
CONTRAINDICATIONS
Prior hypersensitivity reaction to TROGARZO or any components of the product. (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions including infusion-related reactions and anaphylactic reactions have been reported following infusion of TROGARZO. (5.1)
- Immune Reconstitution Inflammatory Syndrome (IRIS) has been reported in patients treated with combination antiretroviral therapies. (5.2)
- Embryo-Fetal Toxicity: Monitor infants exposed to TROGARZO in utero for signs and symptoms of immunosuppression. (5.3, 8.1)
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 5%) were diarrhea, dizziness, nausea, and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact THERA patient support® at 1-833-23THERA (1-833-238-4372) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Lactation: Women infected with HIV should be instructed not to breastfeed due to the potential for HIV transmission. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Including Infusion-Related and Anaphylactic Reactions
5.2 Immune Reconstitution Inflammatory Syndrome
5.3 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage regimen is a single loading dose of 2,000 mg followed by a maintenance dose of 800 mg every 2 weeks administered as a diluted intravenous infusion (IV infusion) or undiluted intravenous push (IV push) [see Dosage and Administration (2.2, 2.3)]. TROGARZO is available in a single-dose, 2 mL vial containing 150 mg/mL of ibalizumab-uiyk. Each vial delivers approximately 1.33 mL containing 200 mg of ibalizumab-uiyk. Dose modifications of TROGARZO are not required when administered with any other antiretroviral or any other treatments.
2.2 Preparation
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard vial if solution is cloudy, if there is pronounced discoloration or if there is foreign particulate matter.
See Table 1 for the appropriate number of vials required to prepare both the loading dose of 2,000 mg and the maintenance doses of 800 mg.
Table 1. Recommended TROGARZO Dose and Number of Vials Per Administration TROGARZO Dose TROGARZO Vials
(Total Volume to be Withdrawn)Loading dose of 2,000 mg
(IV infusion or undiluted IV push)
10 vials (13.3 mL) Maintenance dose of 800 mg
(IV infusion or undiluted IV push)
4 vials (5.32 mL) TROGARZO solution for IV infusion or IV push should be prepared by a trained medical professional using aseptic technique as follows:
For intravenous infusion
For administration as an IV infusion, the appropriate number of vials are diluted in 250 mL of 0.9% Sodium Chloride Injection, USP.
• Remove the flip-off cap from the single-dose vial and wipe the stopper with an alcohol swab.
• Insert sterile syringe needle into the vial through the center of the stopper and withdraw 1.33 mL from each vial (NOTE: a small residual amount may remain in the vial, discard unused portion) and transfer into a 250 mL intravenous bag of 0.9% Sodium Chloride Injection, USP. Other intravenous diluents must not be used to prepare the TROGARZO solution for infusion.
• Once diluted, the TROGARZO solution should be administered immediately.
• If not administered immediately, store the diluted TROGARZO solution at room temperature (20°C to 25°C, 68°F to 77°F) for up to 4 hours, or refrigerated (2°C to 8°C, 36°F to 46°F) for up to 24 hours. If refrigerated, allow the diluted TROGARZO solution to stand at room temperature (20°C to 25°C, 68°F to 77°F) for at least 30 minutes but no more than 4 hours prior to administration.
• Discard partially used vials or empty vials of TROGARZO and any unused portion of the diluted TROGARZO solution.
For intravenous push
For administration as an IV push, undiluted TROGARZO solution is administered.
• Allow the vials to stand at room temperature for approximately 5 minutes.
• Remove the flip-off cap from the single-dose vial and wipe the stopper with an alcohol swab.
• Insert sterile syringe needle into the vial through the center of the stopper and withdraw 1.33 mL from each vial (NOTE: a small residual amount may remain in the vial, discard unused portion).
• The undiluted TROGARZO solution should be administered immediately.
• Discard partially used vials or empty vials of TROGARZO and any unused portion of the undiluted TROGARZO solution.
2.3 Administration
TROGARZO solution should be administered by a trained medical professional.Administer TROGARZO intravenously (as an IV infusion or IV push) in the cephalic vein of the patients right or left arm. If this vein is not accessible, an appropriate vein located elsewhere can be used. Table 2 outlines the duration of IV infusion or IV push for the loading dose and maintenance dose.
Table 2. Duration of IV Infusion or IV Push IV Infusion (Diluted) IV Push (Undiluted) Loading Dose 2,000 mg Over at least 30 minutes Over at least 90 seconds Maintenance Dose 800 mg Over at least 15 minutes Over at least 30 seconds
After the IV infusion is complete, flush with 30 mL of 0.9% Sodium Chloride Injection, USP.
After the IV push is complete, flush with 2 to 5 mL of 0.9% Sodium Chloride Injection, USP.
All patients must be observed for 1 hour after completion of TROGARZO loading dose as an IV infusion or IV push. If the patient does not experience an infusion-associated adverse reaction, the post-administration observation time for the subsequent maintenance doses (IV infusion or IV push) can be reduced to 15 minutes.
If a maintenance dose (800 mg) of TROGARZO is missed by 3 days or longer beyond the scheduled dosing day, a loading dose (2,000 mg) should be administered as early as possible. Resume maintenance dosing (800 mg) every 14 days thereafter. - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
TROGARZO is contraindicated in patients with a prior hypersensitivity reaction to TROGARZO or any components of the product [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Including Infusion-Related and Anaphylactic Reactions
Hypersensitivity reactions including infusion-related reactions and anaphylactic reactions have been reported following infusion of TROGARZO during post-approval use. Symptoms may include dyspnea, angioedema, wheezing, chest pain, chest tightness, cough, hot flush, nausea, and vomiting. If signs and symptoms of an anaphylactic or other clinically significant hypersensitivity reaction occur, immediately discontinue administration of TROGARZO and initiate appropriate treatment. The use of TROGARZO is contraindicated in patients with known hypersensitivity with TROGARZO [see Contraindications (4), Adverse Reactions (6.2)].
5.2 Immune Reconstitution Inflammatory Syndrome
Immune reconstitution inflammatory syndrome has been reported in one patient treated with TROGARZO in combination with other antiretrovirals. During the initial phase of combination antiretroviral therapies, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections, which may necessitate further evaluation and treatment.
5.3 Embryo-Fetal Toxicity
Based on animal data, TROGARZO may cause reversible immunosuppression (CD4+ T cell and B cell lymphocytopenia) in infants born to mothers exposed to TROGARZO during pregnancy. Immune phenotyping of the peripheral blood and expert consultation are recommended to provide guidance regarding monitoring and management of exposed infants based on the degree of immunosuppression observed. The safety of administering live or live-attenuated vaccines in exposed infants is unknown. [see Use In Specific Populations (8.1)]
-
6 ADVERSE REACTIONS
The following adverse drug reactions are discussed in other sections of the labeling:
- Immune Reconstitution Inflammatory Syndrome [see Warnings and Precautions (5.2)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 350 subjects have been exposed to TROGARZO in the ibalizumab clinical development program, including 45 subjects who received TROGARZO through expanded access programs. A total of 19 subjects received TROGARZO via IV push. The safety profile of TROGARZO administered via IV push (Trial TMB-302) was similar to that seen with IV infusion administration (Trial TMB-301) [see Clinical Pharmacology (12.3)].
Trial TMB-301
The primary safety assessment of TROGARZO is based on 24 weeks of data from Trial TMB-301. TMB-301 was a single-arm trial of TROGARZO which enrolled 40 heavily treatment-experienced subjects with multidrug resistant HIV-1 on a failing HIV treatment regimen. Subjects received a single 2,000 mg IV loading dose of TROGARZO followed seven days later by the initiation of an optimized background regimen (OBR) including at least one agent to which the subject's virus was susceptible. Two weeks after the TROGARZO loading dose, 800 mg of TROGARZO was administered IV. The IV administration of TROGARZO 800 mg was continued every 2 weeks through Week 25.The most common adverse reactions (all Grades) reported in at least 5% of subjects were diarrhea, dizziness, nausea, and rash. Table 3 shows the frequency of adverse reactions occurring in 5% or more of subjects.
Table 3. Adverse Reactions (All Grades) Reported in ≥ 5% of Subjects Receiving TROGARZO and Optimized Background Regimen for 23 Weeks in Trial TMB-301 - *
- Includes pooled terms “rash”, “rash erythematous”, “rash generalized”, “rash macular”, “rash maculopapular”, and “rash papular”
% Subjects
N=40Diarrhea 8% Dizziness 8% Nausea 5% Rash* 5% Most (90%) of the adverse reactions reported were mild or moderate in severity. Two subjects experienced severe adverse reactions: one subject had a severe rash and one subject developed immune reconstitution inflammatory syndrome manifested as an exacerbation of progressive multifocal leukoencephalopathy.
Laboratory Abnormalities
Table 4 shows the frequency of laboratory abnormalities (≥ Grade 3) in Trial TMB-301.Table 4. Selected Laboratory Abnormalities (≥ Grade 3) in Trial TMB-301 % Subjects
N=40
Bilirubin (≥ 2.6 x ULN)
5%
Direct Bilirubin (> ULN)
3%
Creatinine (> 1.8 x ULN or 1.5 x baseline)
10%
Blood Glucose (> 250 mg/dL)
3%
Lipase (> 3.0 x ULN)
5%
Uric Acid (> 12 mg/dL)
3%
Hemoglobin (< 8.5 g/dL)
3%
Platelets (< 50,000/mm3)
3%
Leukocytes (< 1.5 x 109 cells/L)
5%
Neutrophils (< 0.6 x 109 cells/L)
5%
6.2 Postmarketing Experience
The following adverse reactions have been identified during post‐approval use of TROGARZO. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Immune system disorders: hypersensitivity reactions including infusion-related reactions and anaphylactic reactions have been reported [see Warnings and Precautions (5.1)].
- Skin and subcutaneous tissue disorders: pruritus
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiretrovirals during pregnancy. This registry does not include Trogarzo, but likely includes patients’ concomitant antiretroviral drugs. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1–800–258–4263.
Risk Summary
Based on animal data, ibalizumab-uiyk use during pregnancy may cause reversible immunosuppression (CD4+ T cell and B cell lymphocytopenia) in infants exposed to ibalizumab-uiyk in utero. Immunoglobulin G (IgG) antibodies, such as ibalizumab-uiyk, are transported across the placenta in significant amounts, especially near term; therefore, ibalizumab-uiyk has the potential to be transferred from the mother to the developing fetus (see Clinical Considerations). There are no available data on ibalizumab-uiyk use in pregnant women to evaluate for a drug- associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
In a reproductive study in monkeys, reversible decreases in CD4+ T cells and B cells and increases in CD8+ T cells were observed within the first 4 weeks after birth in infants born to pregnant monkeys receiving ibalizumab-uiyk intravenously (see Data). Lymphocyte counts returned to near normal levels by 3 months of age. One infant monkey died from a systemic viral infection that may be related to ibalizumab-uiyk-induced immunosuppression. No malformations or premature births were observed in this study.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Immunoglobulin G (IgG) antibodies are increasingly transported across the placenta as pregnancy progresses, with the largest amount transferred during the third trimester. Administration of TROGARZO during pregnancy may affect immune responses in the in utero-exposed infant. For infants with perinatal exposure to TROGARZO, immune phenotyping of the peripheral blood, including CD4+ T cell and B cell counts, is recommended. Expert consultation is also recommended to provide guidance on monitoring and management (e.g., need for antibiotic or immunoprophylaxis) of exposed infants based on the degree of immunosuppression observed. The safety of administering live or live-attenuated vaccines in exposed infants is unknown.
Animal Data
In an enhanced pre- and post-natal development (ePPND) study, pregnant cynomolgus monkeys were administered intravenous doses of either vehicle or 110 mg/kg ibalizumab-uiyk every week from Gestation Day 20-22 (GD 20-22) until parturition on GD 160 ± 10. Significant changes in infant monkey immune cell levels on Postnatal Day (PND) 14 (mean decreases of 78% in CD4+ T cells and 46% in B cells and increases of 2.3-fold in CD8+ T cells) and PND 28 (mean decreases of 73% in CD4+ T cells and increases of 2.2-fold in CD8+ T cells), attributed to in utero ibalizumab-uiyk exposure, were observed relative to concurrent controls. The lymphocyte changes correlated with infant ibalizumab-uiyk serum concentrations and appeared to return to near normal levels between PND 28-91, when ibalizumab-uiyk concentrations were nearly undetectable. Although ibalizumab-uiyk exposure in these infant monkeys may be significantly higher than in human infants following in utero exposure at the recommended human maintenance dose, the risk of ibalizumab-uiyk-induced immunosuppression in human infants is possible. No meaningful differences in infant monkey lymphocyte counts were observed on PND 180. Further, no differences in immune cell function were observed in a T cell-dependent response assay conducted on PND 138 to 180 ± 2 following immunization of the infant monkeys with keyhole limpet hemocyanin. One treatment-group infant monkey died on PND 24 from a systemic viral infection with secondary superficial bacterial infection which was acquired during the postnatal period. Despite the low incidence (1 of 20 infants), the death may be related to ibalizumab-uiyk-induced immunosuppression. Decreases in CD4+ T cells (93%), and B cells (92%) were observed in this infant on PND 14, and decreased cellularity was observed in the spleen, thymus and mandibular lymph node. Unlike the rest of the ibalizumab-exposed infant monkey population, this infant also exhibited a decrease in CD8+ T cells of 71% on PND 14. Body weight was also decreased in this infant between PND 14 and 24. No structural abnormalities were observed among the ibalizumab-uiyk-exposed infants. In addition, no maternal toxicities, including no changes in maternal lymphocyte subsets or effects on embryo-fetal survival, were observed.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV-1-infected mothers in the United States not breastfeed their infants to avoid the risk of postnatal transmission of HIV-1 infection.No data are available regarding the presence of TROGARZO in human milk, the effects on the breastfed child, or the effects on milk production. Human IgG is present in human milk, although published data indicate that antibodies in breast milk do not enter the neonatal or infant circulation system in substantial amounts. Because of the potential for HIV-1 transmission, instruct mothers not to breastfeed if they are receiving TROGARZO.
-
11 DESCRIPTION
TROGARZO is a CD4-directed post-attachment HIV-1 inhibitor.
Ibalizumab-uiyk is a CD4 domain 2-directed humanized monoclonal antibody of immunoglobulin G (IgG) isotype 4 with a molecular weight of approximately 150 kDa. Ibalizumab-uiyk is produced by recombinant DNA technology in murine myeloma non-secreting 0 (NS0) cells.
TROGARZO Injection is a sterile, colorless to slightly yellow and clear to slightly opalescent solution with no visible particles in a single-dose vial for intravenous administration (by IV infusion or IV push). Each single-dose vial delivers approximately 1.33 mL containing 200 mg of ibalizumab-uiyk, and contains the following inactive ingredients: 10 mM L-histidine (2.06 mg), 0.045% polysorbate 80 (0.60 mg), 52 mM sodium chloride (4.04 mg) and 5.2% sucrose (69.2 mg). TROGARZO solution has a pH of 6.0 and contains no preservative.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
A clear trend was identified between exposure and response rate for the Phase 2b trial (TMB-202) which studied two different intravenous doses given at two different dosing intervals (every 4 weeks vs. every 2 weeks). The recommended intravenous dosing regimen consisting of a 2,000 mg loading dose followed by a maintenance dose of 800 mg every 2 weeks was selected on the basis of these results.
12.3 Pharmacokinetics
Ibalizumab-uiyk administered as a single agent exhibits nonlinear pharmacokinetics. Following single-dose administrations of ibalizumab-uiyk as 0.5 to 1.5-hour infusions, the area under the concentration-time curve increased in a greater than dose-proportional manner, clearance decreased from 9.54 to 0.36 mL/h/kg and elimination half-life increased from 2.7 to 64 hours as the dose increased from 0.3 to 25 mg/kg. The volume of distribution of ibalizumab-uiyk was approximately that of serum volume, at 4.8 L.
Following the recommended dose regimen (a single loading dose of 2,000 mg followed by a maintenance dose of 800 mg every 2 weeks), ibalizumab-uiyk concentrations reached steady-state levels after the first 800 mg maintenance dose with mean concentrations over 30 mcg/mL throughout the dosing interval. Among HIV-infected subjects, the pharmacokinetic data from Trial TMB-302 demonstrated that ibalizumab-uiyk Ctrough and AUC0-tau values between IV push administration and IV infusion administration for maintenance dose were similar. The Cmax values were 25% higher for IV push compared to the IV infusion administration. The increase in Cmax is not considered clinically relevant.
The initial loading dose of ibalizumab-uiyk as an IV push administration over 90 seconds is predicted to have a similar Cmax and AUC relative to IV infusion administration over 30 minutes.
Specific Populations
A population pharmacokinetic analysis was performed to explore the potential effects of selected covariates (age, body weight, sex, baseline CD4+ cell count) on ibalizumab-uiyk pharmacokinetics. The result suggests that ibalizumab-uiyk concentration decreases as body weight increases; however, the effect is unlikely to impact virologic outcome and does not warrant a dose adjustment.Pediatric/Geriatric Patients: Ibalizumab-uiyk pharmacokinetics have not been evaluated in pediatric or geriatric patients [see Use in Specific Populations (8.4, 8.5)].
Renal/Hepatic Impairment: No formal studies were conducted to examine the effects of either renal or hepatic impairment on the pharmacokinetics of ibalizumab-uiyk. Renal impairment is not anticipated to impact the pharmacokinetics of ibalizumab-uiyk.
Drug Interaction studies
No drug interaction studies have been conducted with ibalizumab-uiyk. Based on ibalizumab-uiyk’s mechanism of action and target-mediated drug disposition, drug-drug interactions are not expected.12.4 Microbiology
Mechanism of Action
Ibalizumab-uiyk, a recombinant humanized monoclonal antibody, blocks HIV-1 from infecting CD4+ T cells by binding to domain 2 of CD4 and interfering with post-attachment steps required for the entry of HIV-1 virus particles into host cells and preventing the viral transmission that occurs via cell-cell fusion.Ibalizumab-uiyk Does Not Impact CD4 Function
The binding specificity of ibalizumab-uiyk to domain 2 of CD4 allows ibalizumab-uiyk to block viral entry into host cells without causing immunosuppression. Epitope mapping studies indicate that ibalizumab-uiyk binds to a conformational epitope located primarily in domain 2 of the extracellular portion of the CD4 receptor. This epitope is positioned on the surface of CD4 opposite to the site in domain 1 that is required for CD4 binding of the MHC class II molecules and therefore does not interfere with CD4-mediated immune functions. Additionally, ibalizumab-uiyk does not interfere with gp120 attachment to CD4.Antiviral Activity
Ibalizumab-uiyk inhibits the replication of CCR5- and CXCR4-tropic laboratory strains and primary isolates of HIV-1 in phytohemagglutinin stimulated peripheral blood lymphocytes. The median EC50 value (50% effective concentration) for ibalizumab-uiyk against HIV-1 group M isolates (subtypes A, B, C, D, E, or O) was 8 ng/mL (n = 15, range of 0.4 to 600 ng/mL) in cell culture, with lower susceptibility observed in macrophage-tropic HIV-1 strains (BaL, JR-CSF, YU2, and ADA-M). In a single-cycle infection assay, ibalizumab-uiyk inhibited 17 clinical isolates of subtype B with a median EC50 value of 12 ng/mL (range of 8.8 to 16.9 ng/mL; mean 12 ± 3 ng/mL) and a median maximum percentage inhibition (MPI) of 97% (range of 89 to 99%; mean 97 ± 3%). Three CCR5-tropic clinical isolates from subtypes B, C, and D, were inhibited with EC50 values ranging from 59-66 ng/mL and 3 CXCR4-tropic clinical isolates from subtypes B, C, and D, with EC50 values ranging from 44-59 ng/mL.Antiviral Activity in Combination with Other Antiviral Agents
No antagonism was observed when PBMCs or MAGI-CCR5 cells infected with the subtype B Ba-L or ADA variants of HIV-1 were incubated with ibalizumab-uiyk in combination with the CCR5 co-receptor antagonist maraviroc or when PBMCs infected with the subtype B HT/92/599 variant of HIV-1 were incubated with ibalizumab-uiyk in combination with the gp41 fusion inhibitor enfuvirtide; a nonnucleoside reverse transcriptase inhibitor (efavirenz); nucleoside analog reverse transcriptase inhibitors (abacavir, didanosine, emtricitabine, tenofovir, or zidovudine); or a protease inhibitor (atazanavir).Antiviral Activity in Antiretroviral-Resistant Virus
Subjects enrolled in TMB-301 were heavily treatment-experienced subjects infected with multidrug resistant HIV-1. Ibalizumab-uiyk inhibited 38 baseline isolates at a median EC50 value of 31 ng/mL (range of 13 to 212 ng/mL; mean 39 ± 35 ng/mL) with a median MPI of 97% (range of 41-100%; mean 91 ± 14%). For 10 subjects in TMB-301 who failed treatment, at the time of failure the median ibalizumab-uiyk EC50 value was 566 ng/mL (range of 148 to >54,900 ng/mL; mean 11,768 ± 21,650 ng/mL) representing an EC50 value shift of >18-fold. For the HIV-1 derived from the same subjects, the median MPI was 55% (range of 43-72%; mean 56 ± 8%) representing a 42 percentage point reduction.Decreased Susceptibility
Decreased susceptibility to ibalizumab-uiyk, as defined by a decrease in MPI, has been observed in some subjects experiencing virologic failure and may be associated with genotypic changes in the HIV-1 envelope coding sequence that results in the loss of potential N-linked glycosylation sites (PNGS) in the V5 loop of gp120. The clinical significance of decreased susceptibility to ibalizumab-uiyk has not been established.Cross-Resistance
Phenotypic and genotypic test results revealed no evidence of cross-resistance between ibalizumab-uiyk and any of the approved classes of anti-retroviral drugs (CCR5 co-receptor antagonists, gp41 fusion inhibitors, integrase strand transfer inhibitors [INSTIs], non-nucleos(t)ide reverse transcriptase inhibitors [NNRTIs], nucleos(t)ide reverse transcriptase inhibitors [NRTIs], or protease inhibitors [PIs]). Ibalizumab-uiyk is active against HIV-1 resistant to all approved antiretroviral agents and exhibits antiretroviral activity against R5-tropic, X4-tropic, and dual-tropic HIV-1.Decreased susceptibility to ibalizumab-uiyk following multiple dose administrations of ibalizumab-uiyk has been observed in some subjects. Cell culture studies performed with HIV-1 variants with reduced susceptibility to ibalizumab-uiyk indicate that phenotypic changes associated with resistance to ibalizumab-uiyk do not alter susceptibility to other approved agents and do not result in the selection of CD4-independent viral isolates.
CD4 Polymorphisms and Ibalizumab-uiyk Activity
CD4 polymorphisms reported in public databases were analyzed to determine if any naturally occurring amino acid substitutions in the CD4 molecule from different human populations would potentially impact the antiviral activity of ibalizumab-uiyk. None of the known CD4 polymorphisms are likely to have an impact on ibalizumab-uiyk binding to CD4.12.6 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to ibalizumab-uiyk in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
All subjects enrolled in clinical trial TMB-301 and trial TMB-202 (a Phase 2b clinical trial that studied TROGARZO administered intravenously as 2,000 mg every 4 weeks or 800 mg every 2 weeks; the safety and effectiveness of this dosing regimen has not been established), were tested for the presence of anti-ibalizumab antibodies throughout their participation. One sample tested positive with low titer anti-ibalizumab antibodies. No adverse reaction or reduced efficacy was attributed to the positive sample reported in this subject. - 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
Trial TMB-301:
Trial TMB-301 was a single arm, multicenter clinical trial conducted in 40 heavily treatment-experienced HIV-infected subjects with multidrug resistant HIV-1. Subjects were required to have a viral load greater than 1,000 copies/mL and documented resistance to at least one antiretroviral medication from each of three classes of antiretroviral medications as measured by resistance testing. Subjects must have been treated with antiretrovirals for at least 6 months and be failing or had recently failed (i.e., in the last 8 weeks) therapy.The trial was composed of three discrete periods:
- Control period (Day 0 to Day 6): Subjects were either monitored on their current failing therapy or received no therapy if they had failed and discontinued treatment within the 8 weeks preceding screening. This was an observational period to establish baseline HIV viral load.
- Functional monotherapy period (Day 7 to Day 13): All subjects received a 2,000 mg loading dose of TROGARZO on Day 7. Subjects on a failing ART regimen continued to receive their failing regimen in addition to the loading dose of TROGARZO. This period was to establish the virologic activity of TROGARZO.
- Maintenance period (Day 14 to Week 25): On Day 14 of the treatment period, viral load was assessed for the primary endpoint, and thereafter the background regimen was optimized to include at least one drug to which the subjects virus was susceptible. The use of an investigational drug(s) as a component of the optimized background regimen was allowed. Beginning at Day 21, an 800 mg maintenance dose of TROGARZO was administered every two weeks through Week 25. This period was to establish the safety and durability of virologic suppression of TROGARZO when used in combination with an optimized background regimen.
The majority of subjects in Trial TMB-301 were male (85%), white (55%) and between 23 and 65 years of age (mean [SD] age: 50.5 [11.0] years). At Baseline, median viral load and CD4+ T cell counts were 35,350 copies/mL and 73 cells/mm3, respectively. The subjects were heavily treatment-experienced: 53% of participants had been treated with 10 or more antiretroviral drugs prior to trial enrollment; 98% percent had been treated with NRTIs, 98% with PIs, 80% with NNRTIs, 78% with INSTIs, 30% with gp41 fusion inhibitors, and 20% with CCR5 co-receptor antagonists.
The primary efficacy endpoint was the proportion of subjects achieving a 0.5 log10 decrease in viral load from the beginning to the end of the "Functional monotherapy period" as compared to the proportion of subjects achieving a 0.5 log10 decrease from the beginning to the end of the "Control period", as defined above. The results of the primary endpoint analysis are shown in Table 5 below.
Table 5. Proportion of Subjects Achieving a 0.5 log10 Decrease in Viral Load at the End of the Control and Functional Monotherapy Periods - *
- exact 95% confidence interval.
p < 0.0001 based on McNemars test comparing the proportion of subjects achieving 0.5 log10 decrease in viral load at the end of the control and functional monotherapy periods.
Proportion of Subjects Achieving a 0.5 log10 Decrease in Viral Load
N=4095% CI* End of Control Period 3% (0.06%, 13%) End of Functional Monotherapy Period 83% (67%, 93%) At Week 25, viral load <50 and <200 HIV-1 RNA copies/mL was achieved in 43% and 50% of subjects, respectively. Fifty-five percent of subjects had a 1 log10 reduction in viral load, and 48% of subjects had a 2 log10 reduction in viral load at Week 25. An increase in the mean and median number of CD4+ T-cells (44 cells/mm3 and 17 cells/mm3, respectively) was observed from Baseline to Week 25. Week 25 outcomes are shown in Table 6 and Table 7.
Table 6. Trial TMB 301 Virologic Outcomes (Snapshot Algorithm) at Week 25 - *
- included subjects who had ≥ 50 copies/mL in the Week 25 window, subjects who discontinued study drug due to lack of efficacy, and subjects who discontinued study drug for reasons other than an AE, death and at the time of discontinuation had a viral value ≥ 50 copies/mL
- †
- included subjects who had ≥ 200 copies/mL in the Week 25 window, subjects who discontinued study drug due to lack of efficacy, and subjects who discontinued study drug for reasons other than an AE, death and at the time of discontinuation had a viral value ≥ 200 copies/mL
TROGARZO (N=40) HIV RNA < 50 copies/mL at Week 25
HIV RNA 50 copies/mL at Week 25**43%
45%HIV RNA < 200 copies/mL at Week 25
HIV RNA 200 copies/mL at Week 25†50%
38%No virologic data at Week 25
Discontinued due to AE or death
13%
Table 7. Virologic Response at Week 25 by Baseline CD4 Cell count, Viral Load, Integrase Inhibitor Resistance and OSS * - *
- OSS Overall Susceptibility Score. The OSS indicates the number of fully active drugs in a subjects OBR based on both current and available historical resistance test results. Demonstrating drug susceptibility by both genotypic and phenotypic testing was required, when testing by both methods was technically feasible. As an example, an OSS of 2 would indicate that the HIV-1 isolate tested was fully susceptible to two drugs in the OBR.
Subjects achieving <50 HIV-1 RNA copies/mL (%)
Subjects achieving <200 HIV-1 RNA copies/mL (%)
CD4 Cell Counts
<50 (n=17)
50-200 (n=10)
>200 (n=13)
18
60
62
24
70
69
Viral Load
100,000 (n=33)
>100,000 (n=7)
49
14
58
14
Resistance
With INSTI Resistance (n=27)
Without INSTI Resistance (n=13)
41
46
44
62
OSS
0 (n=5)
1 (n=12)
2 (n=18)
3 (n=3)
4 (n=2)
20
42
50
33
50
20
50
61
33
50
-
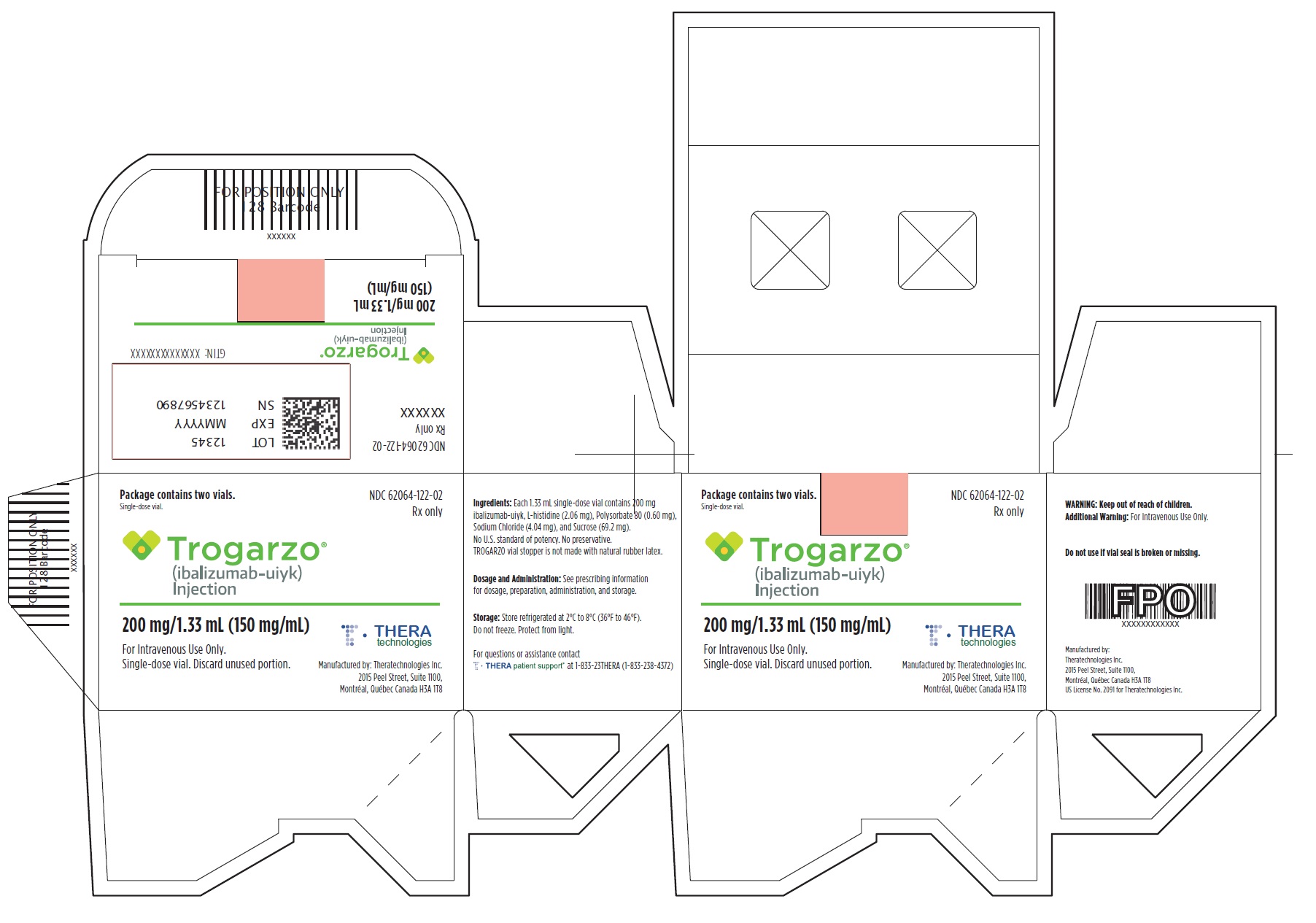
16 HOW SUPPLIED/STORAGE AND HANDLING
TROGARZO (ibalizumab-uiyk) injection is a sterile colorless to slightly yellow and clear to slightly opalescent solution with no visible particles for intravenous administration (by IV infusion or IV push). It is packaged in a single-dose 2 mL clear glass vial containing 200 mg/1.33 mL (150 mg/mL) of ibalizumab-uiyk. TROGARZO vial stopper is not made with natural rubber latex.
TROGARZO is available in a carton containing two single-dose vials (NDC 62064-122-02).
Store vials under refrigeration at 2 to 8ºC (36-46 ºF). Do not freeze and protect from light.
Once diluted, the TROGARZO solution should be administered immediately [see Dosage and Administration (2.2)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity
Advise patients of the risk of hypersensitivity reactions including anaphylaxis. Instruct patients to seek immediate medical attention if signs or symptoms of hypersensitivity occur or are suspected. Advise patients who have had clinically significant hypersensitivity reactions to TROGARZO that they should not receive TROGARZO [see Contraindications (4), Warnings and Precautions (5.1)].Immune Reconstitution Syndrome
Immune Reconstitution Inflammatory Syndrome: Advise patients that immune reconstitution syndrome has been reported in a patient receiving TROGARZO and to inform their health care provider immediately of any symptoms of infection [see Warnings and Precautions (5.2)].Important Administration Information
Advise the patient it is important to receive TROGARZO injections every two weeks as recommended by their healthcare professional and not to change the dosing schedule of TROGARZO or any antiretroviral medication without consulting their healthcare provider. Advise the patient to contact their healthcare provider immediately if they stop taking TROGARZO or any other drug in their antiretroviral regimen [see Dosage and Administration (2)]. Advise the patient that they may receive TROGARZO loading dose by IV infusion over at least 30 minutes or by IV push over 90 seconds and they may receive TROGARZO maintenance doses by IV infusion over 15 minutes or IV push over 30 seconds. Also, advise the patient to seek counsel of the healthcare provider regarding the most appropriate route of administration [see Dosage and Administration (2.3)].Embryo-fetal Toxicity
Advise pregnant individuals and females of reproductive potential of the potential risk of reversible immunosuppression in infants exposed to TROGARZO during pregnancy and to inform their healthcare provider of a known or suspected pregnancy [see Warning and Precautions (5.3) and Use in Specific Populations (8.1)].Lactation
Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in breast milk [see Use in Specific Populations (8.2)]. -
PATIENT PACKAGE INSERT
PATIENT INFORMATION
TROGARZO® (tro-gar-zo)
(ibalizumab-uiyk)
injectionWhat is TROGARZO?
TROGARZO is a prescription medicine that is used with other antiretroviral medicines to treat Human Immunodeficiency Virus-1 (HIV-1) infection in adults who:- have received several anti-HIV-1 regimens in the past, and
- have HIV-1 virus that is resistant to many antiretroviral medicines, and
- who are failing their current antiretroviral therapy
HIV-1 is the virus that causes Acquired Immune Deficiency Syndrome (AIDS).
It is not known if TROGARZO is safe and effective in children.Do not receive TROGARZO if you have had an allergic reaction to TROGARZO or any of the ingredients in TROGARZO. See the end of this leaflet for a complete list of ingredients in TROGARZO.
Before you receive TROGARZO, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if TROGARZO may harm your unborn baby. Tell your healthcare provider if you become pregnant during treatment with TROGARZO. Pregnancy Registry: There is a pregnancy registry for women who take antiretroviral medicines during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk with your healthcare provider about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. Do not breastfeed if you are receiving TROGARZO.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- It is not known if TROGARZO passes into breast milk.
Talk with your healthcare provider about the best way to feed your baby during treatment with TROGARZO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive TROGARZO?
- You will receive TROGARZO by your healthcare provider as an intravenous infusion (IV infusion) or as an intravenous push (IV push). The first dose will be given over 30 minutes by IV infusion or 90 seconds by IV push. After the first dose, the dose will be given over 15 minutes by IV infusion or 30 seconds by IV push. A healthcare provider will monitor you during the TROGARZO IV infusion or IV push and for a period of time after your IV infusion or IV push.
- You will receive TROGARZO every two weeks.
- It is important that you receive TROGARZO every two weeks as instructed by your healthcare provider. Do not change the schedule of your TROGARZO IV infusion or IV push, or any of your antiretroviral medicines without talking to your healthcare provider first.
- Tell your healthcare provider right away if you stop receiving TROGARZO or stop taking any other antiretroviral medicines.
What are the possible side effects of TROGARZO?
TROGARZO can cause serious side effects, including:- Allergic reactions. TROGARZO can cause allergic reactions, including serious reactions, during and after IV infusion or IV push. Tell your healthcare provider or nurse, or get medical help right away if you get any of the following symptoms of an allergic reaction:
o trouble breathing
o cough
o swelling in your throat
o hot flush
o wheezing
o nausea
o chest pain
o vomiting
o chest tightness-
Changes in your immune system (Immune Reconstitution Inflammatory Syndrome) can happen when you start taking HIV-1 medicines. Your immune system might get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you start having new symptoms after receiving TROGARZO.
The most common side effects of TROGARZO include:
- diarrhea
- nausea
- dizziness
- rash
These are not all of the possible side effects of TROGARZO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to
 at 1-833-23THERA (1-833-238-4372).
at 1-833-23THERA (1-833-238-4372).General information about the safe and effective use of TROGARZO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your healthcare provider for information about TROGARZO that is written for health professionals.
What are the ingredients in TROGARZO?
Active ingredient: ibalizumab-uiyk
Inactive ingredients: L-histidine, polysorbate 80, sodium chloride, and sucrose.TROGARZO does not contain any preservative. TROGARZO vial stopper is not made with natural rubber latex.
Manufactured by Theratechnologies Inc., 2015 Peel Street, Suite 1100, Montréal, Québec Canada H3A 1T8
US License No. 2091 for Theratechnologies Inc.For more information, call
 1-833-23THERA (1-833-238-4372) or go to www.TROGARZO.com.
1-833-23THERA (1-833-238-4372) or go to www.TROGARZO.com.
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 12/2023
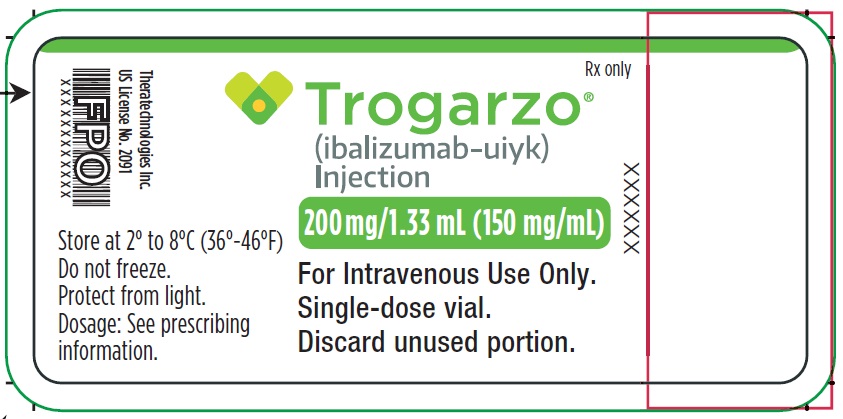
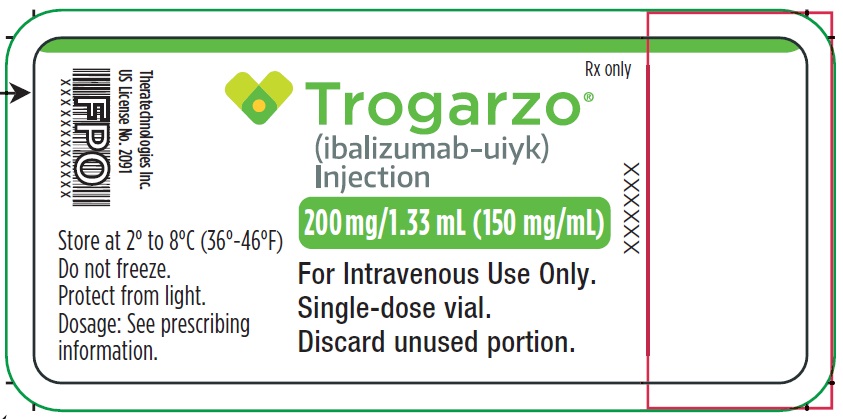
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Trogarzo Carton Label
Package contains two vials.
Single-dose vial.
NDC 62064-122-02
Rx only
Trogarzo®
(ibalizumab-uiyk)
Injection200 mg/1.33 mL (150 mg/mL)
For Intravenous Use Only.
Single-dose vial. Discard unused portion.
Manufactured by: Theratechnologies Inc.
2015 Peel Street, Suite 1100,
Montréal, Québec Canada H3A 1T8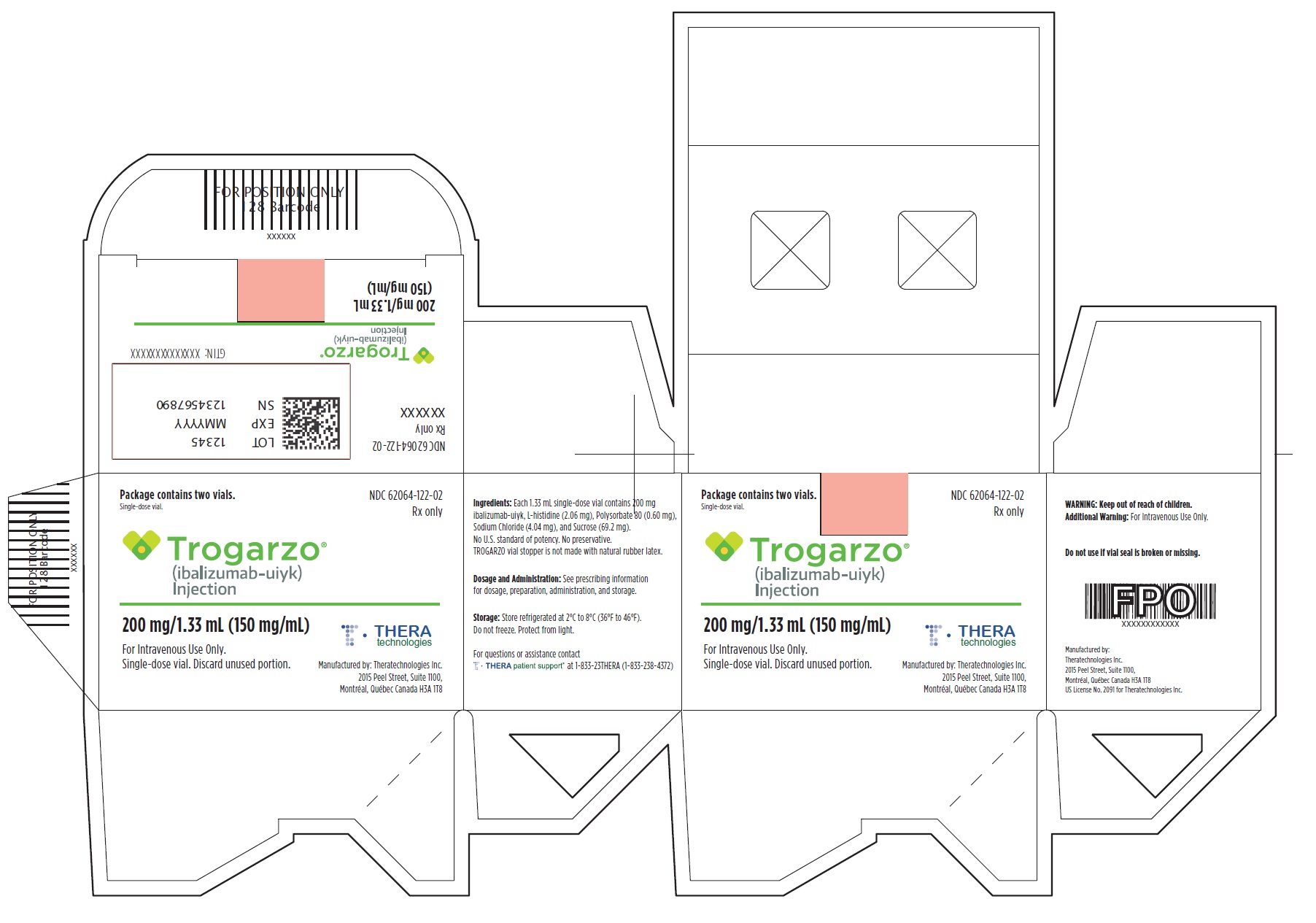
-
INGREDIENTS AND APPEARANCE
TROGARZO
ibalizumab injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62064-122 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ibalizumab (UNII: LT369U66CE) (ibalizumab - UNII:LT369U66CE) ibalizumab 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) sucrose (UNII: C151H8M554) sodium chloride (UNII: 451W47IQ8X) polysorbate 80 (UNII: 6OZP39ZG8H) HYDROCHLORIC ACID (UNII: QTT17582CB) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62064-122-02 2 in 1 CARTON 04/20/2018 1 NDC:62064-122-01 1.33 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761065 04/20/2018 Labeler - Theratechnologies Inc. (252017520) Registrant - Theratechnologies Inc. (252017520)