Label: DERMELEVE ANTI ITCH SCALP SERUM- aluminum acetate solution
- NDC Code(s): 81507-004-01
- Packager: Advanced Derm Solutions LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
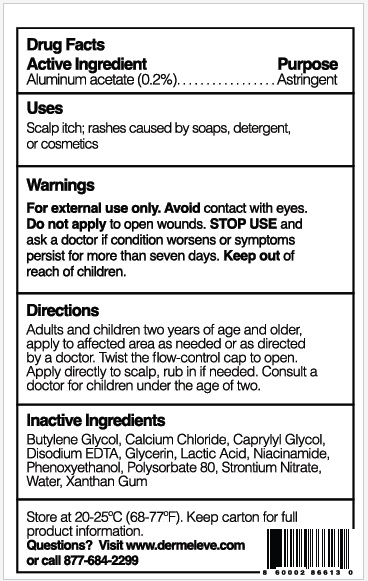
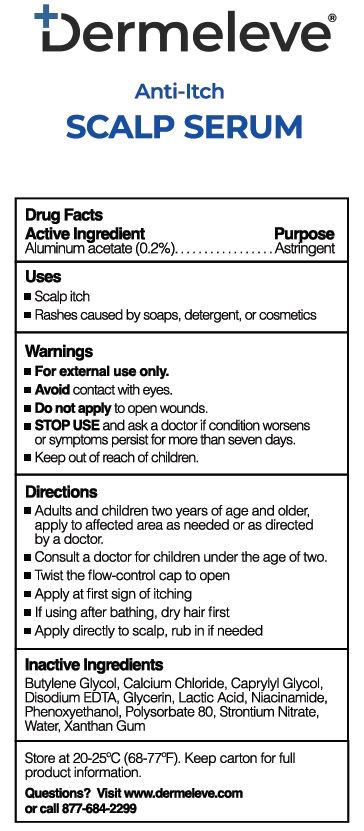
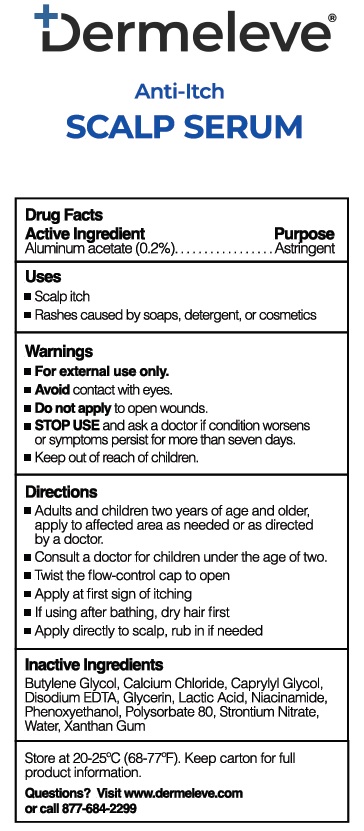
- Active Ingredient
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- Inactive ingredients
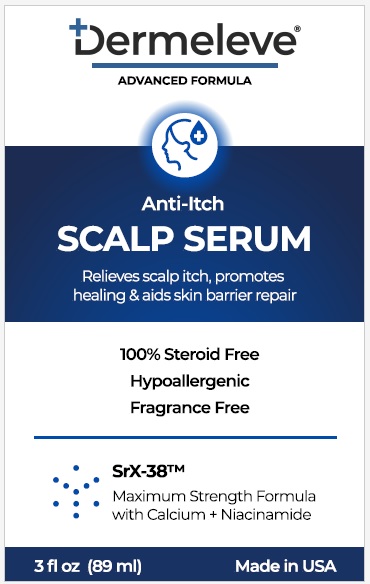
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DERMELEVE ANTI ITCH SCALP SERUM
aluminum acetate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81507-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ACETATE (UNII: 80EHD8I43D) (ALUMINUM CATION - UNII:3XHB1D032B) ALUMINUM ACETATE 0.2 g in 100 mL Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) CAPRYLYL GLYCOL (UNII: 00YIU5438U) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GLYCERIN (UNII: PDC6A3C0OX) LACTIC ACID (UNII: 33X04XA5AT) NIACINAMIDE (UNII: 25X51I8RD4) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STRONTIUM NITRATE (UNII: BDG873AQZL) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81507-004-01 89 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/02/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/02/2023 Labeler - Advanced Derm Solutions LLC (117840544)