Label: BRIGHTEN LIGHTENING- hydroquinone gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 54272-301-11 - Packager: CEN BEAUTY LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 5, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
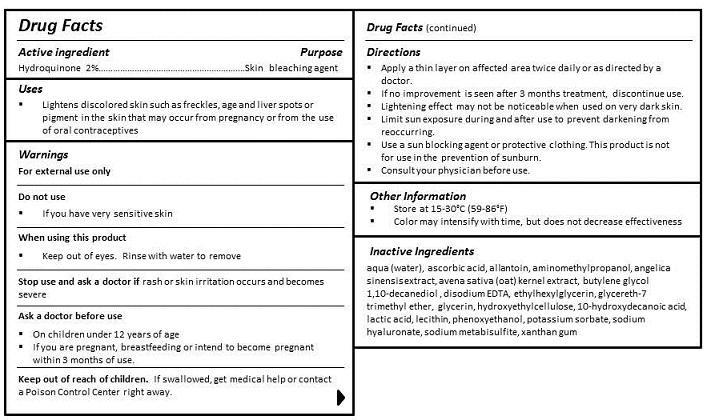
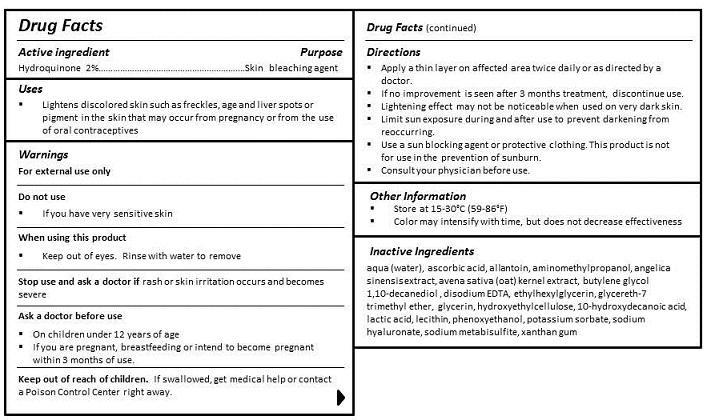
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- ASK DOCTOR
-
DOSAGE & ADMINISTRATION
DIRECTIONS
- Apply a thin layer on affected area twice daily or as directed by a doctor.
- If no improvement is seen after 3 months treatment, discontinue use.
- Lightening effect may not be noticeable when used on very dark skin.
- Limit sun exposure during and after use to prevent darkening from reoccurring.
- Use a sun blocking agent or protective clothing. This product is not for use in the prevention of sunburn.
- Consult your physician before use.
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
INGREDIENTS
aqua (water), cyclopentasiloxane, PEG-10 dimethicone, dimethicone, butylene glycol, cyclohexasiloxane, glycerin, carollina officinalis extract, algae extract, avena sativa (oat) kernel extract, angelica sinensis extract, 10-hydroxydecanoic acid, sebacic acid, 1,10-decanediol acid, betaine, dimethicone/vinyl dimethicone crosspolymer, lauryl PEG-9 polydimethylsiloxyethyl dimethicone, lecithin, dimethicone/PEG-10/15 crosspolymer, triethoxycaprylylsilane, decamethylcyclopentasiloxane, trifluoromethyl C1-C4 alkyl dimethicone, quaternium-90, bentonite, butylene glycol, sodium chloride, sodium citrate, ethylhexylglycerin, propylene carbonate, propylene glycol, potassium sorbate, methylisothiazolinone, iodopropynyl butylcarbamate, phenoxyethanol
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BRIGHTEN LIGHTENING
hydroquinone gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54272-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ASCORBIC ACID (UNII: PQ6CK8PD0R) ALLANTOIN (UNII: 344S277G0Z) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) GLYCERIN (UNII: PDC6A3C0OX) OAT (UNII: Z6J799EAJK) ANGELICA SINENSIS WHOLE (UNII: 697D19QDBN) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 10-HYDROXYDECANOIC ACID (UNII: NP03XO416B) 1,10-DECANEDIOL (UNII: 5I577UDK52) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM (UNII: 7FLD91C86K) LACTIC ACID (UNII: 33X04XA5AT) GLYCERETH-7 TRIMETHYL ETHER (UNII: XMC7402M60) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM METABISULFITE (UNII: 4VON5FNS3C) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54272-301-11 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part358A 02/21/2013 Labeler - CEN BEAUTY LLC (078664118) Registrant - CEN BEAUTY LLC (078664118)