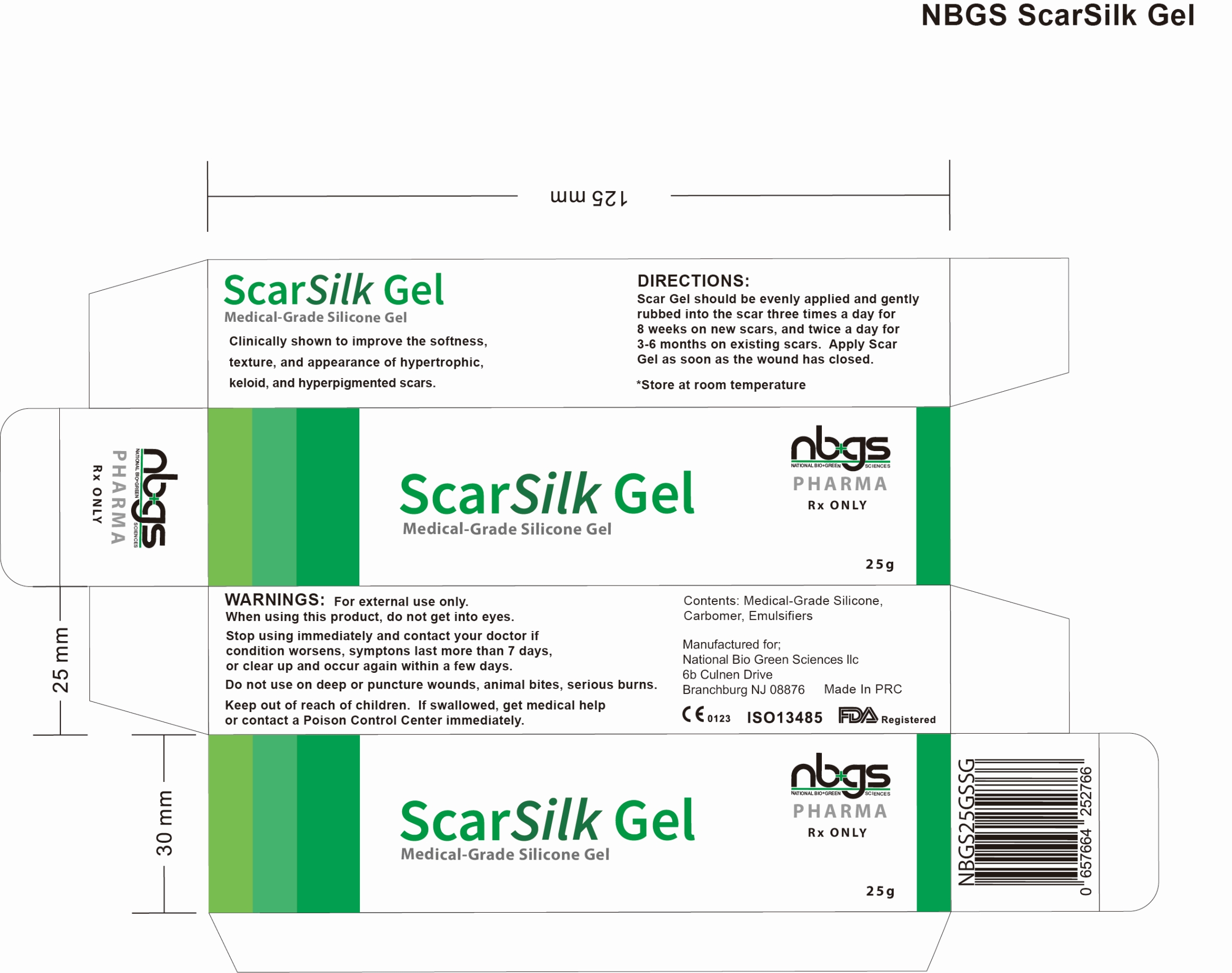
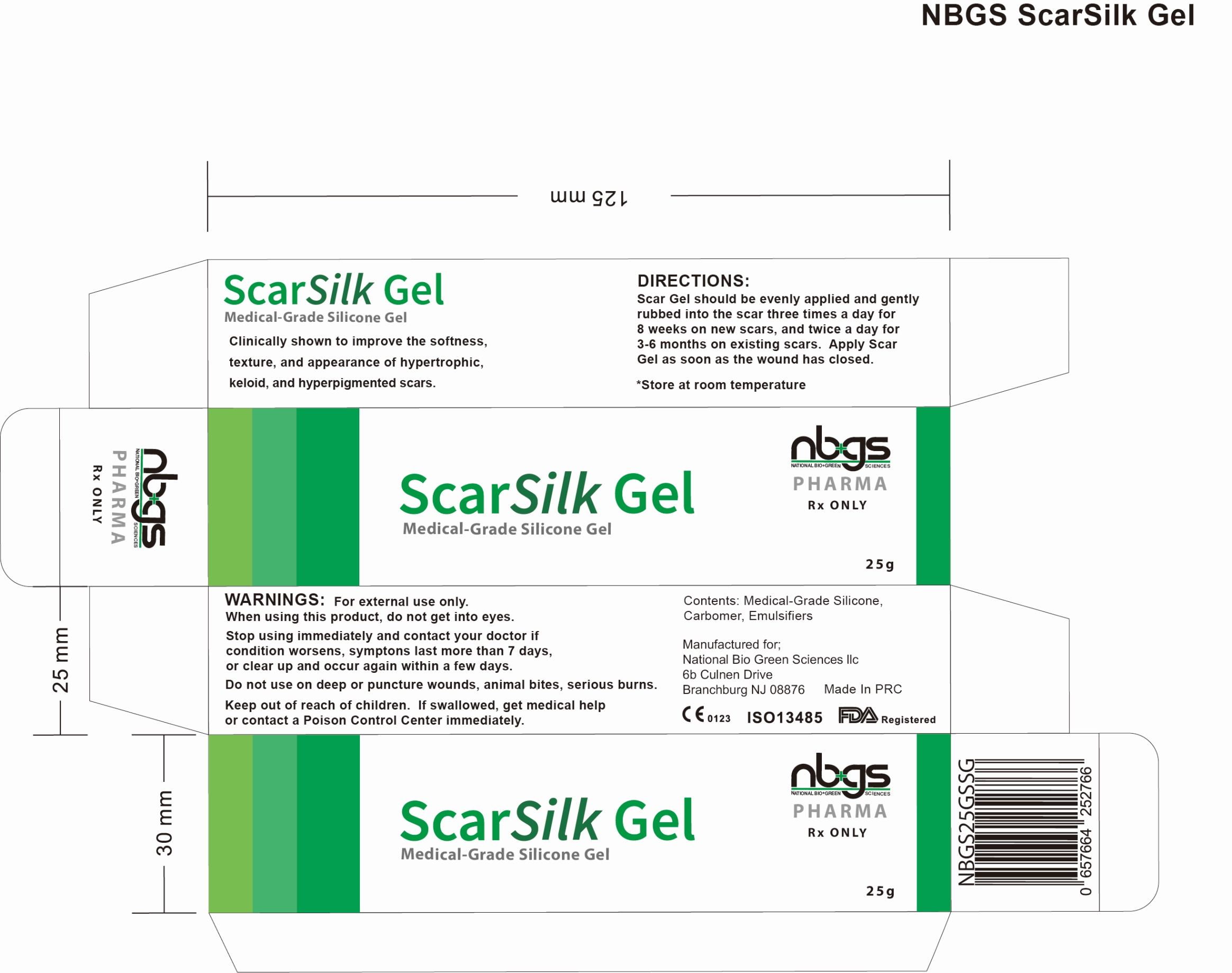
Label: NBGS SCARSILK MEDICAL GRADE SILICONE SCAR GEL-
- NHRIC Code(s): 72678-002-01
- Packager: NATIONAL BIO GREEN SCIENCES LIMITED LIABILITY COMPANY
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: Exempt device
Drug Label Information
Updated July 15, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
nbgs ScarSilk Gel is intended for use in the management of closed hyperproliferative scars, both old and new hypertrophic or keloid scars resulting from burns, surgical procedures or trauma wounds. It helps reduce redness, softens and flattens raised scars, relieves itching, discomfort and pain associated with scars, helps prevent excessive and abnormal scar formation, and for use as mono-therapy or in combination with other scar therapies including pressure garments.
nbgs ScarSilk Gel is a self-drying, flexible, gas permeable, waterproof silicone gel that is colorless and odorless. SCARSilk GEL forms a bond with the stratum corneum (the outer layer of dead skin cells) forming a protective barrier against chemical physical and microbial invasion of the scar site while assisting with hydration.
nbgs ScarSilk Gel is also for use on children or people with sensitive skin. Silicone gel and sheets are internationally recommended as the first line of treatment in scar management - INGREDIENTS
-
CLINCIAL PHARMACOLOGY
The exact mechanism of action in improving the appearance of scar tissue from using silicone remains unknown. However, various suggestions have been made to explain the efficacy of silicone, including hydration, pressure, temperature, oxygen transmission and silicone absorption. There is some evidence that the treatment affects the stratum corneum and, by reducing evaporation, restores better homeostasis in the tissue. In keloid and hypertrophic scarring, the stratum corneum allows more evaporation of water from the underlying tissue than occurs in normal skin. Silicone may prevent this, keeping the stratum corneum in optimal hydration and protecting the skin from environmental hazards, both of which can reduce abnormal scarring. The gel may also affect the stratum corneum by inhibiting mast cell activity, diminishing edema, vasodilatation and excessive extracellular matrix formation but the simple changes in temperature, pressure, oxygen tension and hydration produced by wound coverage probably constitute the main mechanism of action. Another hypothesis is that the effect of static electricity on silicone may influence the alignment of collagen deposition.
- INDICATIONS AND USES
- CONTRAINDICATIONS
-
WARNINGS
For external use only. Avoid direct contact with eyes, lips or mucous membranes. Do not apply on areas of broken skin. Do not apply on third degree burns and open wounds. Never use on sutured wound until sutures have been removed. Do not use on dermatological conditions that disrupt the integrity of the skin.
-
PRECAUTIONS
Stop use and ask a doctor if irritation develops. In rare instances, silicone gel may cause a rash on the skin. This condition may result from improper cleansing of the scar area where the silicone gel has been applied. If this product is applied properly and skin irritation still occurs, discontinue use and consult your physician. If ingested, get medical help or contact Poison Control Center right away.
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
This medication should be used as directed by your physician during pregnancy or while breastfeeding. Consult your doctor about the risks and benefits.
CAUTION: Federal law restricts this device to sale by or on the order of a physician. - ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
1. Ensure that the affected area is clean and dry.
2. Apply ScarSilk Gel to the area as a very thin coat and allow to dry.
3. Apply ScarSilk Gel twice a day
4. Once dry, ScarSilk Gel can be covered with cosmetics or sunscreen.
5. Recommended duration of treatment is 60-90 days.How is the Product Supplied
nbgs ScarSilk Gel is supplied in:
25 gram tubeStore at 20°-25°C (68° to 77°F); Keep away from heat and protect from freezing. [See USP Controlled Room Temperature.]
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NBGS SCARSILK MEDICAL GRADE SILICONE SCAR GEL
elastomer, silicone, for scar managementProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:72678-002 Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) DIMETHICONOL (2000 CST) (UNII: T74O12AN6Y) DIMETHICONE (UNII: 92RU3N3Y1O) CYCLOMETHICONE 4 (UNII: CZ227117JE) SILICON (UNII: Z4152N8IUI) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:72678-002-01 25 in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date exempt device MDA 11/18/2018 Labeler - NATIONAL BIO GREEN SCIENCES LIMITED LIABILITY COMPANY (967054623)