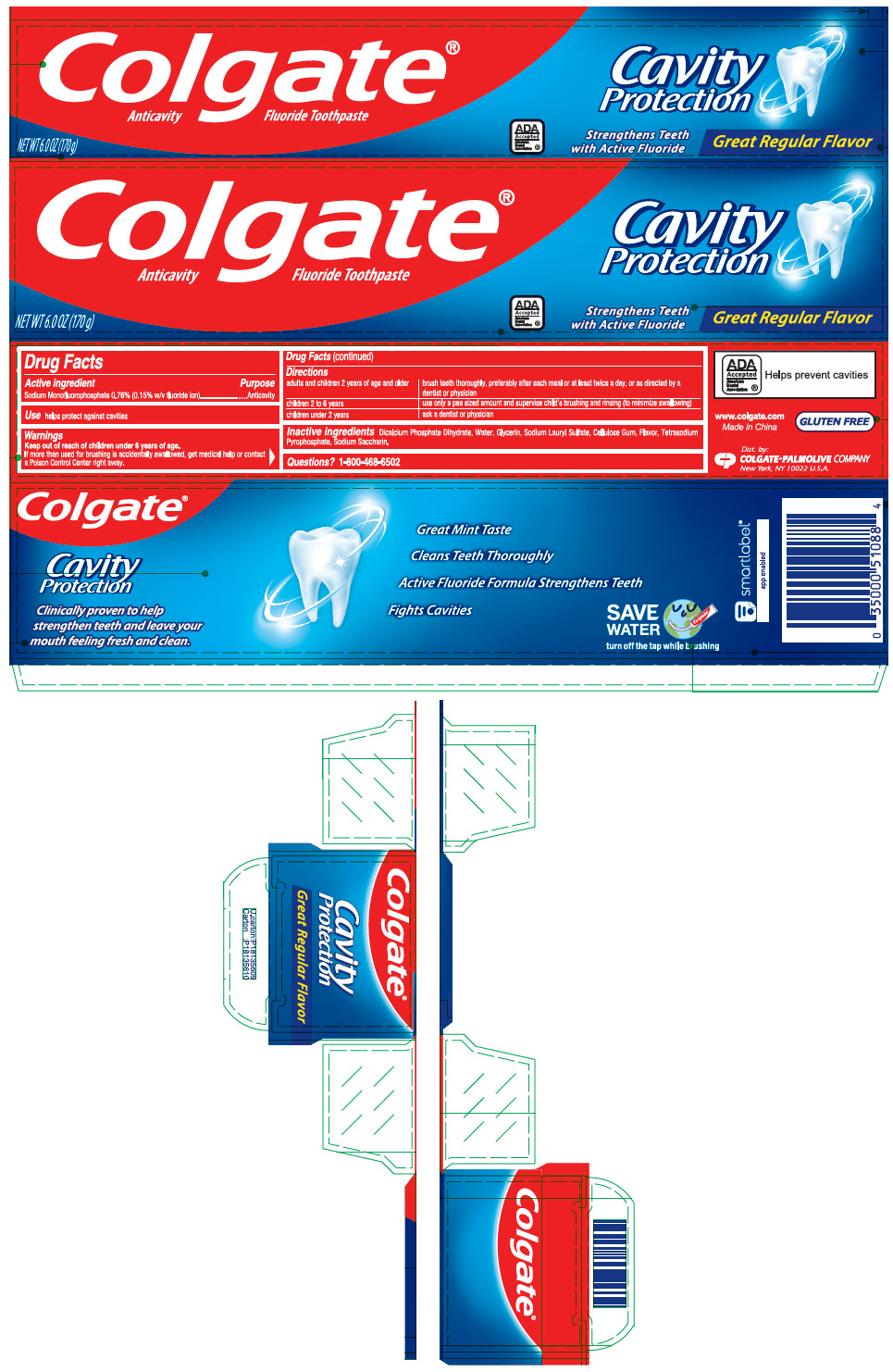
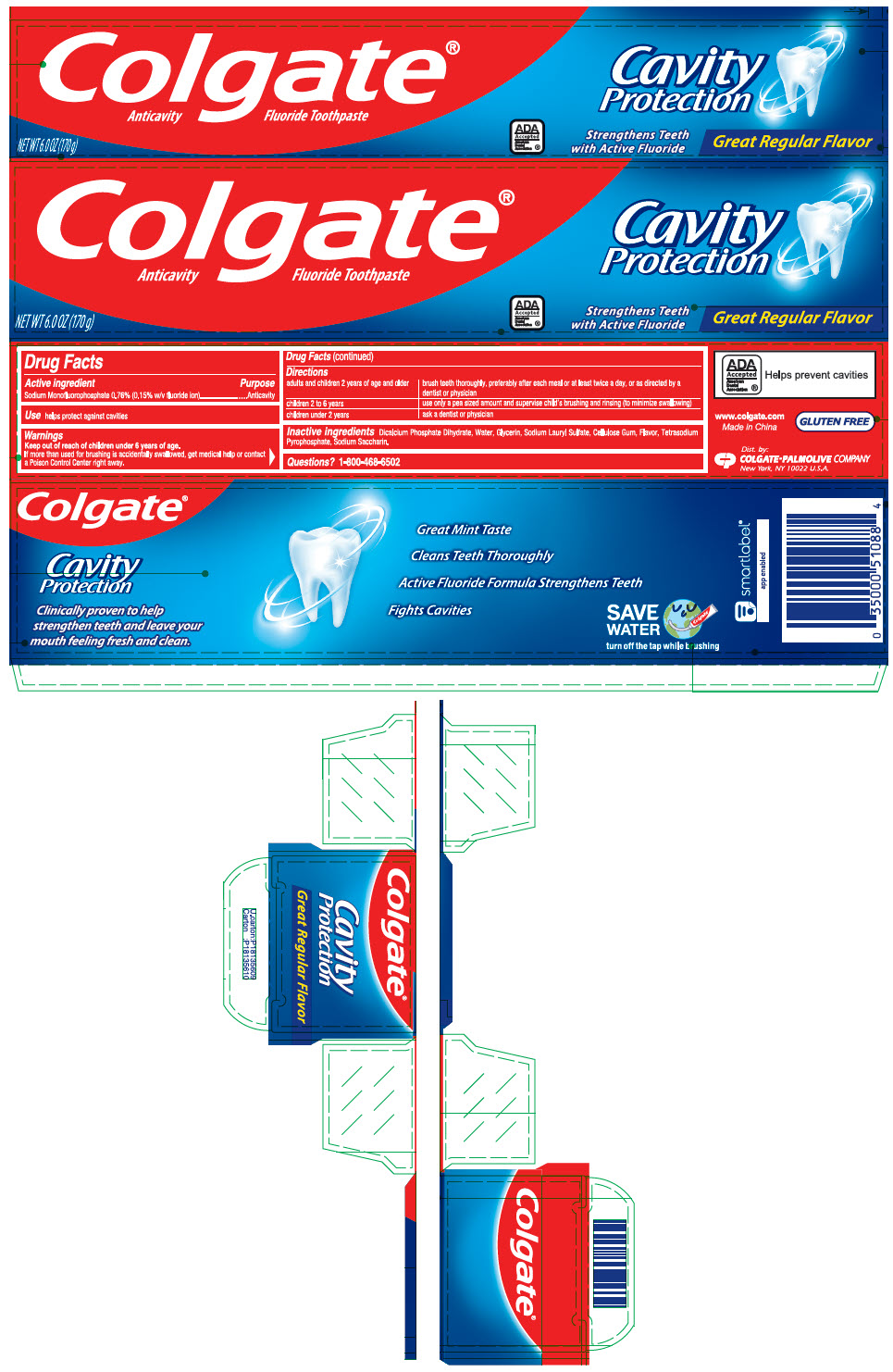
Label: COLGATE GREAT REGULAR FLAVOR- sodium monofluorophosphate paste, dentifrice
- NDC Code(s): 35000-257-01, 35000-257-05, 35000-257-09
- Packager: Colgate-Palmolive Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
adults and children 2 years of age and older brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician children 2 to 6 years use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) children under 2 years ask a dentist or physician - Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 170 g Tube Carton
-
INGREDIENTS AND APPEARANCE
COLGATE GREAT REGULAR FLAVOR
sodium monofluorophosphate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:35000-257 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1 mg in 1 g Inactive Ingredients Ingredient Name Strength DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) SODIUM PYROPHOSPHATE (UNII: O352864B8Z) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color WHITE Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35000-257-01 1 in 1 CARTON 06/18/2022 1 113 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:35000-257-09 1 in 1 CARTON 06/18/2022 2 170 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:35000-257-05 1 in 1 CARTON 06/18/2022 3 141 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part355 06/18/2022 Labeler - Colgate-Palmolive Company (001344381)