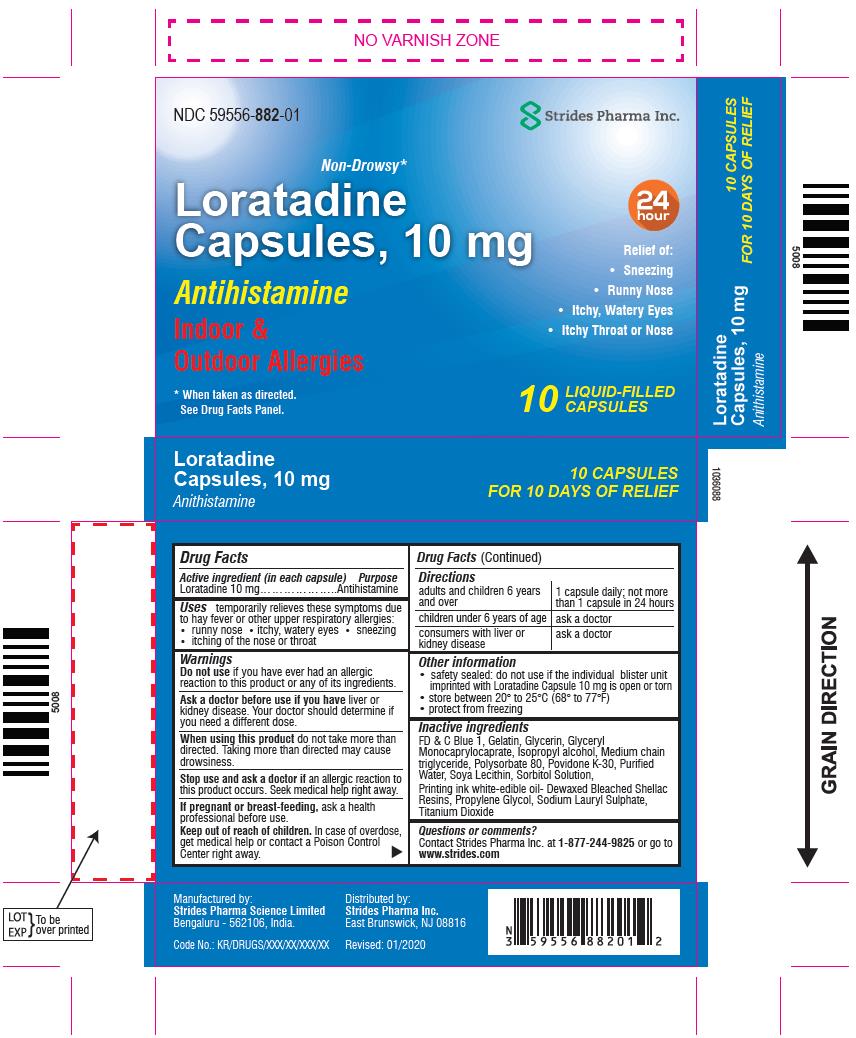
Label: LORATADINE capsule, liquid filled
- NDC Code(s): 59556-882-01, 59556-882-81
- Packager: Strides Pharma Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 28, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each capsule)
- Purpose
- Uses
-
Warnings
OTC - DO NOT USE
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
OTC - ASK DOCTOR
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
OTC - WHEN USING
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
OTC - STOP USE
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
- Directions
- Other information
-
Inactive ingredients
FD & C Blue 1, Gelatin, Glycerin, Glyceryl Monocaprylocaprate, Isopropyl alcohol, Medium chain triglyceride, Polysorbate 80, Povidone K-30, Purified Water, Soya Lecithin, Sorbitol Solution,
Printing ink white-edible oil-Dewaxed Bleached Shellac Resins, Propylene Glycol, Sodium Lauryl Sulphate, Titanium Dioxide
.
- Questions or comments?
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59556-882 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL CAPRYLATE/CAPRATE (UNII: G7515SW10N) ISOPROPYL ALCOHOL (UNII: ND2M416302) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) NONCRYSTALLIZING SORBITOL SOLUTION (UNII: 9E0S3UM200) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE K30 (UNII: U725QWY32X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BLUE Score no score Shape OVAL Size 10mm Flavor Imprint Code 10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59556-882-81 3 in 1 CARTON 06/01/2021 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:59556-882-01 1 in 1 CARTON 06/01/2021 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211926 06/01/2021 Labeler - Strides Pharma Inc (078868278) Registrant - Strides Pharma Science Limited (650738743) Establishment Name Address ID/FEI Business Operations Strides Pharma Science Limited 918513263 ANALYSIS(59556-882) , MANUFACTURE(59556-882) , PACK(59556-882)