Label: LBEL SUPREMACIE NX JOUR REPLENISHING TREATMENT DAYTIME FACE SPF 15 NORMAL TO DRY SKIN- ensulizole, homosalate, octinoxate, oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 43596-0005-0, 43596-0005-1, 43596-0005-2 - Packager: Ventura Corporation, LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 28, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- L'BEL SUPREMACIE NX JOUR Replenishing Treatment Daytime Face Cream SPF 15 Normal To Dry Skin
- Active Ingredients
- Uses
-
Warnings
- Skin Cancer / Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
- For external use only.
- Other Information
-
Inactive Ingredients
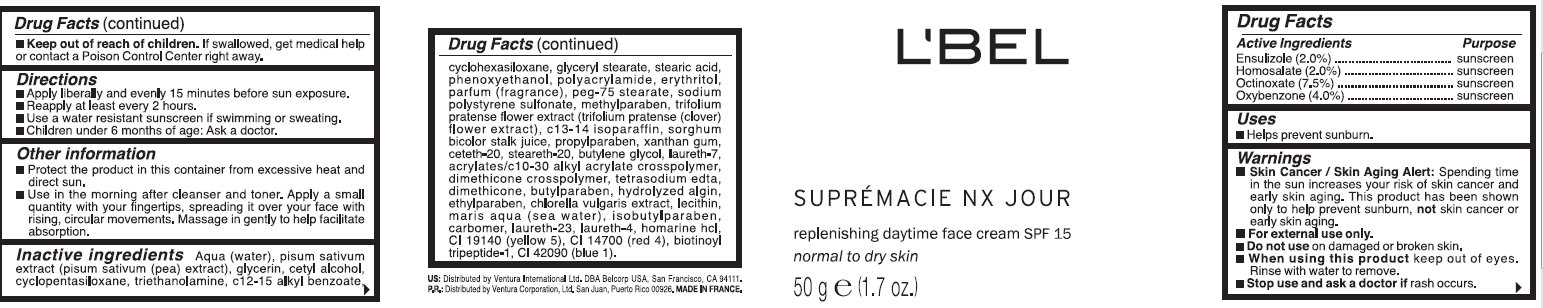
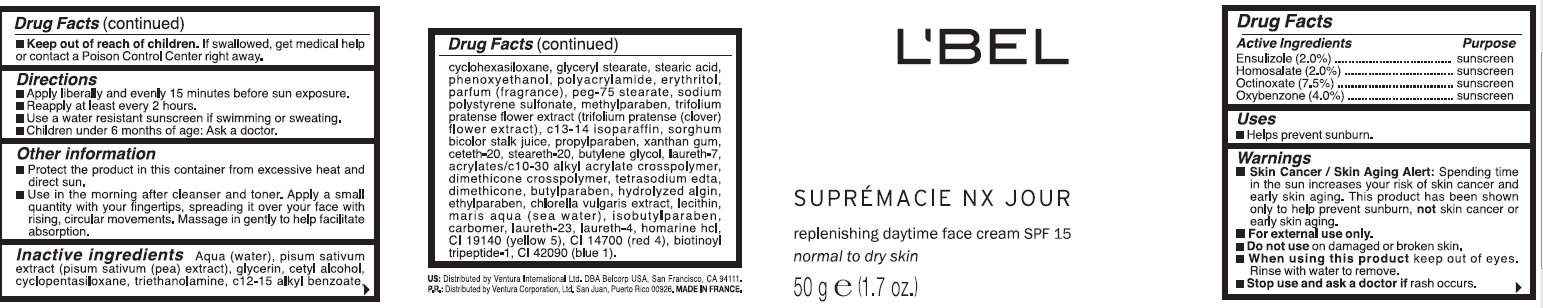
Aqua (water), pisum sativum extract (pisum sativum (pea) extract), glycerin, cetyl alcohol, cyclopentasiloxane, triethanolamine, c12-15 alkyl benzoate, cyclohexasiloxane, glyceryl stearate, stearic acid, phenoxyethanol,polyacrylamide, erythritol,parfum (fragrance), peg-75 stearate, sodium polystyrene sulfonate,methylparaben, trifolium pratense flower extract(trifolium pratense (clover) flower extract), c13-14 isoparaffin,sorghum bicolor stalk juice, propylparaben, xanthan gum, ceteth-20,steareth-20, butylene glycol, laureth-7, acrylates/c10-30 alkyl acrylate crosspolymer, tetrasodium edta, dimethicone, butylparaben, hydrolyzed algin, ethylparaben, chlorella vulgaris extract, lecithin, maris aqua (sea water), isobutylparaben, carbomer, laureth-23, laureth-4, homarine hcl, ci 19140 (yellow 5),ci 14700 (red 4), biotinoyl tripeptide-1, ci 42090 (blue 1).
- Purpose
-
EXPERT ROUTINE L'BEL
L'Bel presents Expert Routine, a skin care program with two steps that work together for enhanced results:
1. Cleanse the skin according to its needs
2. Nourish according to the age.
SUPREMACIE NX JOUR
replenishing daytime face cream SPF 15
L'Bel, fully specialized in cosmetic facial treatment, presents the latest worldwide exclusive: the new Supremacie NX line of replenishing cosmetic treatments improved for women after age 40.
Dazzling beauty for women
The moisturizing formula of Supremacie NX Jour, Offers enhanced results:
- Immediate, strong and long-lasting hydration in the areas where the face needs it most.
- Visibly diminishes the appearance of wrinkles.
- Uncovers skin that looks more firm.
A sensation of well-being that pervades the senses
Its rich and creamy texture, ideal for absorption has been specially created to provide a pleasant sensation of softness and comfort. Its exquisite fragrance rich in essences awakens the skin with new and pleasant emotions.
In two options according to your skin's needs:
- SUPREMACIE NX JOUR - replenishing daytime face cream SPF 15 Normal to Dry Skin
- SUPREMACIE NX JOUR - replenishing daytime face cream SPF 15 Normal to Oily Skin
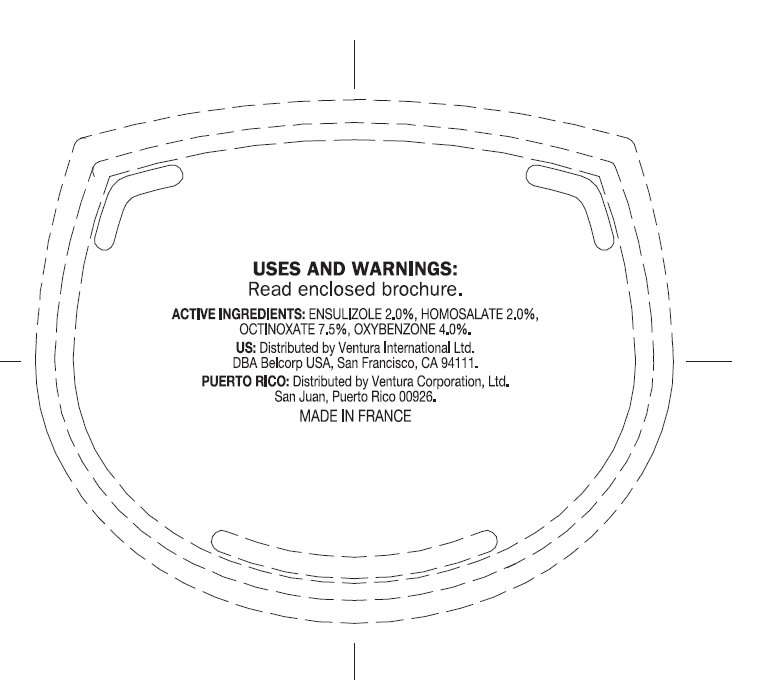
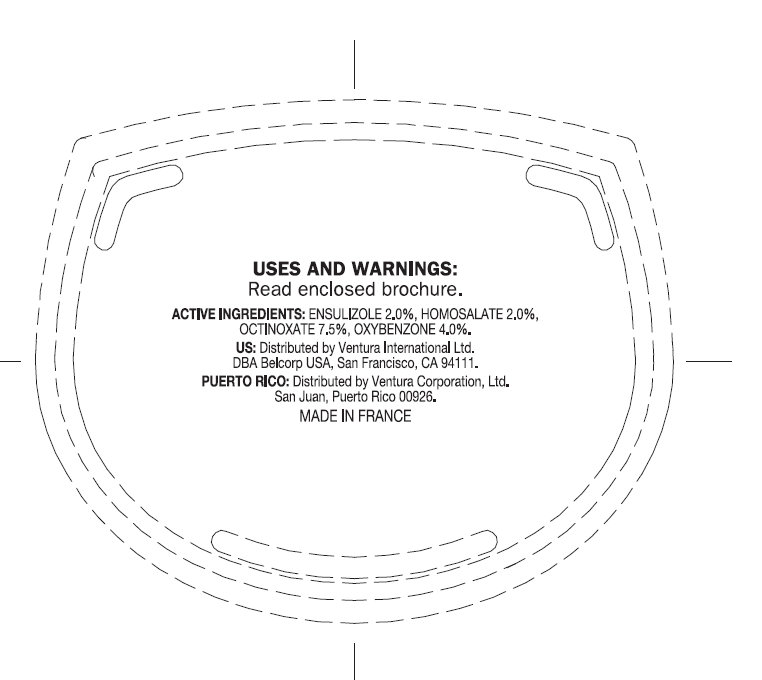
USES: Helps prevent sunburn.
WARNINGS: Skin Cancer / Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging. For external use only. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions: Apply liberally and evenly 15 minutes before sun exposure. Reapply at least every 2 hours.Use a water resistant sunscreen if swimming or sweating. Children under 6 months of age: Ask a doctor.
Other information: Protect the product in this container from excessive heat and direct sun. Use in the morning after cleanser and toner. Apply a small quantity with your fingertips, spreading it over your face with rising, circular movements. Message in gently to help facilitate absorption.
- HYPOALLERGENIC
- DERMATOLOGICALLY AND CLINICALLY TESTED
- PRINCIPAL DISPLAY PANEL
- LBEL SUPREMACIE NX JOUR Replenishing Treatment Daytime Face Cream SPF 15 Normal To Dry Skin
-
INGREDIENTS AND APPEARANCE
LBEL SUPREMACIE NX JOUR REPLENISHING TREATMENT DAYTIME FACE SPF 15 NORMAL TO DRY SKIN
ensulizole, homosalate, octinoxate, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43596-0005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENSULIZOLE (UNII: 9YQ9DI1W42) (ENSULIZOLE - UNII:9YQ9DI1W42) ENSULIZOLE 2 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 2 g in 100 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 4 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PEA (UNII: W4X7H8GYFM) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) TROLAMINE (UNII: 9O3K93S3TK) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CYCLOMETHICONE 6 (UNII: XHK3U310BA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) STEARIC ACID (UNII: 4ELV7Z65AP) PHENOXYETHANOL (UNII: HIE492ZZ3T) ERYTHRITOL (UNII: RA96B954X6) PEG-75 STEARATE (UNII: OT38R0N74H) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) METHYLPARABEN (UNII: A2I8C7HI9T) TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) SORGHUM BICOLOR STEM JUICE (UNII: SKN30A294R) PROPYLPARABEN (UNII: Z8IX2SC1OH) XANTHAN GUM (UNII: TTV12P4NEE) CETETH-20 (UNII: I835H2IHHX) STEARETH-20 (UNII: L0Q8IK9E08) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAURETH-7 (UNII: Z95S6G8201) CARBOMER INTERPOLYMER TYPE A (55000 MPA.S) (UNII: 59TL3WG5CO) EDETATE SODIUM (UNII: MP1J8420LU) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLPARABEN (UNII: 3QPI1U3FV8) METHYLPARABEN (UNII: A2I8C7HI9T) CHLORELLA VULGARIS (UNII: RYQ4R60M02) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) LAURETH-23 (UNII: N72LMW566G) LAURETH-4 (UNII: 6HQ855798J) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 4 (UNII: X3W0AM1JLX) BIOTINOYL TRIPEPTIDE-1 (UNII: O6380721VA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43596-0005-0 1 g in 1 POUCH 2 NDC:43596-0005-1 5 g in 1 JAR 3 NDC:43596-0005-2 50 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/28/2012 Labeler - Ventura Corporation, LTD (602751344) Establishment Name Address ID/FEI Business Operations MF Productions 266769145 manufacture