Label: DARK SPOT CORRECTOR- dark spot corrector cream cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 81940-001-01 - Packager: Shenzhen Weikemo Intelligent Technology CO.,LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 25, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- HOW TO USE?
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
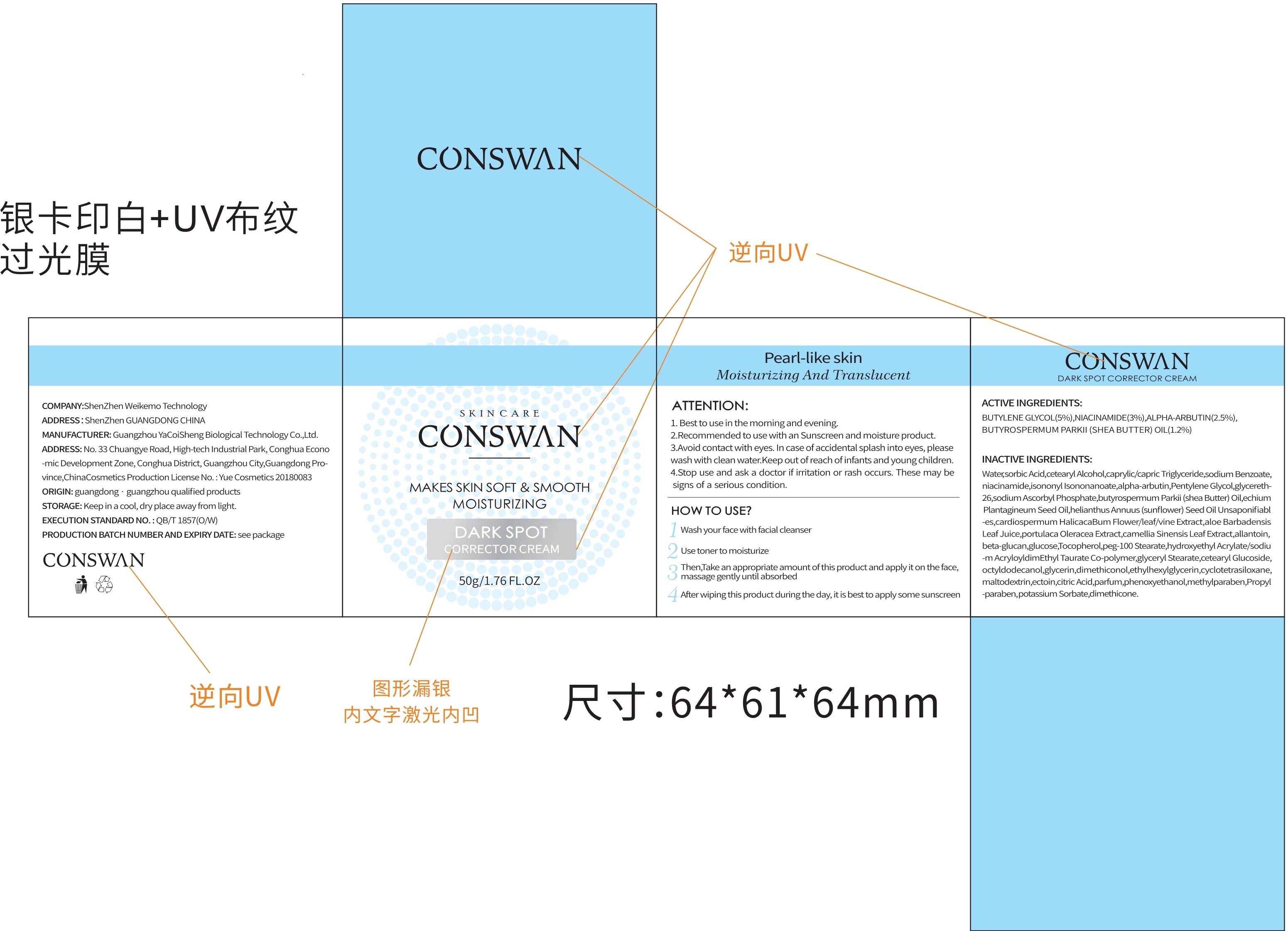
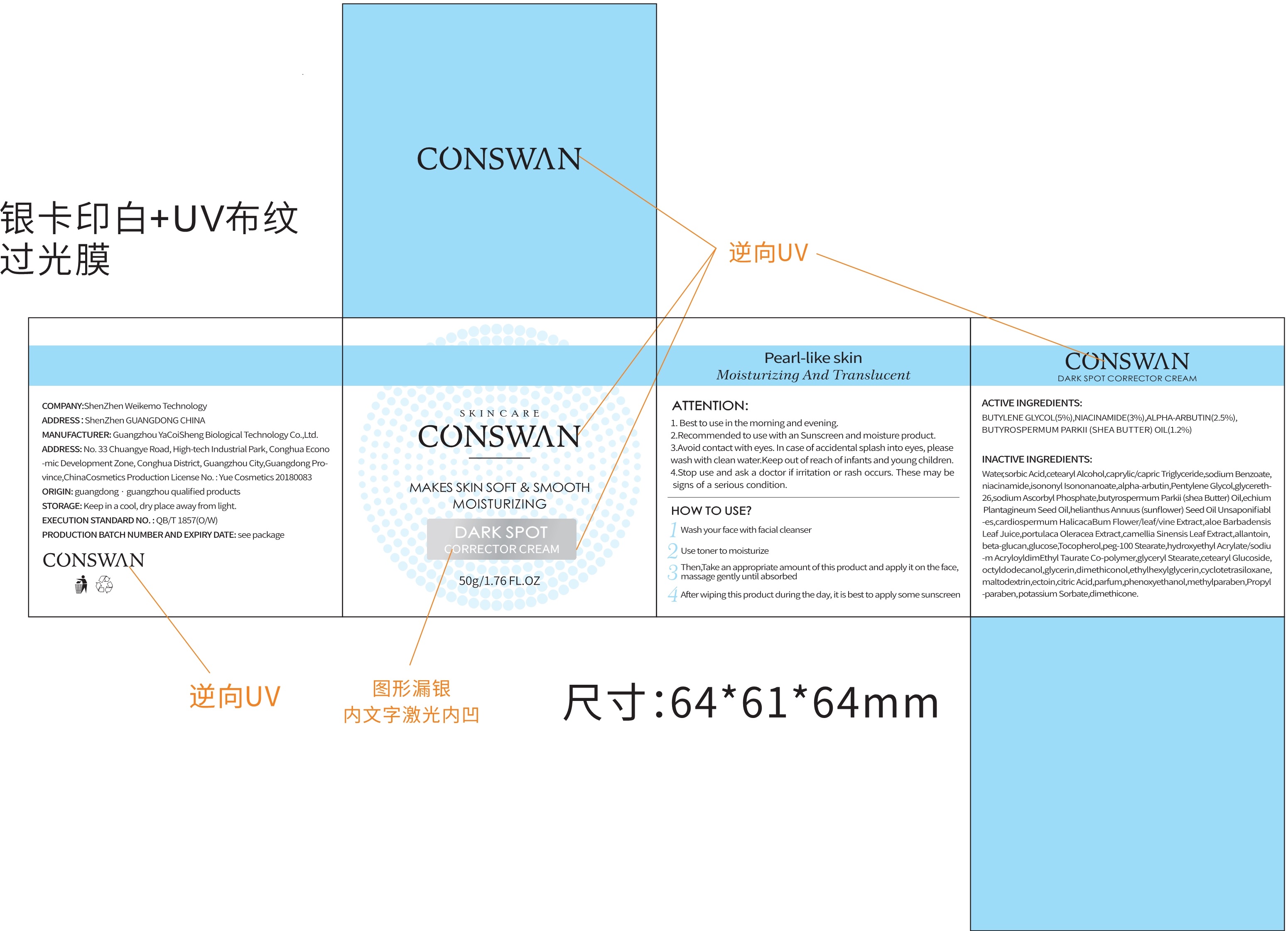
Directions(ATTENTION:)
1.Best to use in the morning and evening.
2.Recommended to use with an Sunscreen and moisture product.
3.Avoid contact with eyes.In case of accidental splash into eyes, please wash with clean water.Keep out of reach of infants and young children.
4.Stop use andask a doctor if irritation or rash occurs.These maybe signs of a serious condition.
- Other information
-
Inactive ingredients
Water, butylene Glycol, dimethicone, cetearyl Alcohol caprylic/capric Triglyceride, niacinamide, isononyl Isononanoate, alpha-ar butin, Pentylene Glycol, glycereth-26, sodium Ascorbyl Phosphate, butyrospermum Parkii(shea Butter) Oil, echium Plan tagineum Seed Oil, helianthus Annuus(sunflower) Seed OilUnsaponifiabl-es, cardiospermumHalicacaBum Flower/leaf/vine Extract, aloe Barbadensis Leaf Juice, portulaca Oleracea Extract, camellia Sinensis Leaf Extract, allantoin, beta-glucan, glucose, Tocopherolpeg-100 Stearate, hydroxyethyl Acrylate/sodiu-m AcryloyldimEthyl Taurate Co-polymer, glyceryl Stearate, cetearyl Glucoside, octyldodecanol, glycerin, dimethiconol, ethylhexyl glycerin, cyclotetra siloxane, maltodextrin, ectoin, citric Acid, parfum, phenoxy ethano, methylparaben, Propyl-paraben, sodium Benzoate, potassium Sorbate, sorbic Acid.
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DARK SPOT CORRECTOR
dark spot corrector cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81940-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 3 g in 100 g ALPHA-ARBUTIN (UNII: 72VUP07IT5) (ALPHA-ARBUTIN - UNII:72VUP07IT5) ALPHA-ARBUTIN 2.5 g in 100 g BUTYLENE GLYCOL (UNII: 3XUS85K0RA) (BUTYLENE GLYCOL - UNII:3XUS85K0RA) BUTYLENE GLYCOL 5 g in 100 g SHEA BUTTER (UNII: K49155WL9Y) (SHEA BUTTER - UNII:K49155WL9Y) SHEA BUTTER 1.2 g in 100 g Inactive Ingredients Ingredient Name Strength ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PENTYLENE GLYCOL (UNII: 50C1307PZG) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) ISOHEXADECANE (UNII: 918X1OUF1E) WATER (UNII: 059QF0KO0R) POLYOXYL 100 STEARATE (UNII: YD01N1999R) CARDIOSPERMUM HALICACABUM FLOWERING TOP (UNII: MZP2508BRR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) TRICAPRIN (UNII: O1PB8EU98M) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM ACRYLOYLDIMETHYLTAURATE (UNII: 2T9Q6EKI0G) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCERIN (UNII: PDC6A3C0OX) GLYCERETH-26 (UNII: NNE56F2N14) CYCLOMETHICONE 4 (UNII: CZ227117JE) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) TEPRALOXYDIM (UNII: 8QHN14BQ7F) CURDLAN (UNII: 6930DL209R) SODIUM BENZOATE (UNII: OJ245FE5EU) ECHIUM PLANTAGINEUM SEED OIL (UNII: PIB7XBU8XW) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONOL (100000 CST) (UNII: OSA9UP217S) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PURSLANE (UNII: M6S840WXG5) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) METHYLPARABEN (UNII: A2I8C7HI9T) SUNFLOWER OIL (UNII: 3W1JG795YI) OCTYLDODECANOL (UNII: 461N1O614Y) ALOE VERA LEAF (UNII: ZY81Z83H0X) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALLANTOIN (UNII: 344S277G0Z) SORBIC ACID (UNII: X045WJ989B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) 2 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81940-001-01 50 g in 1 BOX; Type 0: Not a Combination Product 03/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/30/2020 Labeler - Shenzhen Weikemo Intelligent Technology CO.,LTD (722303220) Establishment Name Address ID/FEI Business Operations Shenzhen Weikemo Intelligent Technology CO.,LTD 722303220 manufacture(81940-001)