Label: LIDOCAINE 4% PATCH- lidocaine 4% patch
- NDC Code(s): 70512-014-30
- Packager: SOLA Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
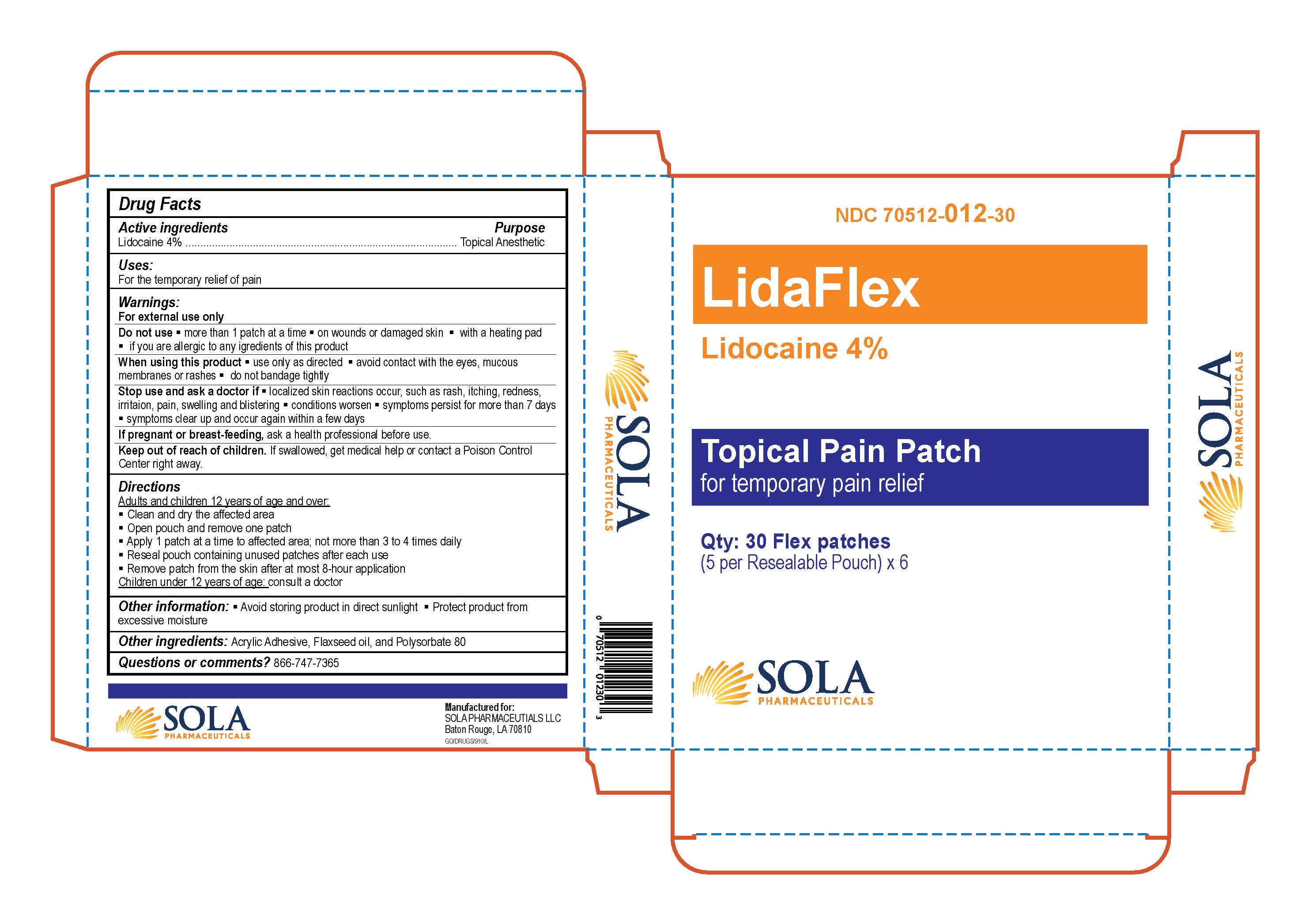
- Active Ingredients
- Purpose
- Uses
- Warnings
- DO NOT USE
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Adults and children 12 years of age or over:
- Clean and dry the affected area
- Open pouch and remove one patch
- Apply 1 patch at a time to affected area; not more than 3 to 4 times daily
- Reseal pouch containing unused patches after each use
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age:
- Consult a doctor
- Other Information
- Other Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOCAINE 4% PATCH
lidocaine 4% patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70512-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength FLAX SEED (UNII: 4110YT348C) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ACRYLIC ACID (UNII: J94PBK7X8S) Product Characteristics Color Score Shape RECTANGLE (White Flexible Patch) Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70512-014-30 6 in 1 BOX 05/24/2021 1 5 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/24/2021 Labeler - SOLA Pharmaceuticals (080121345)