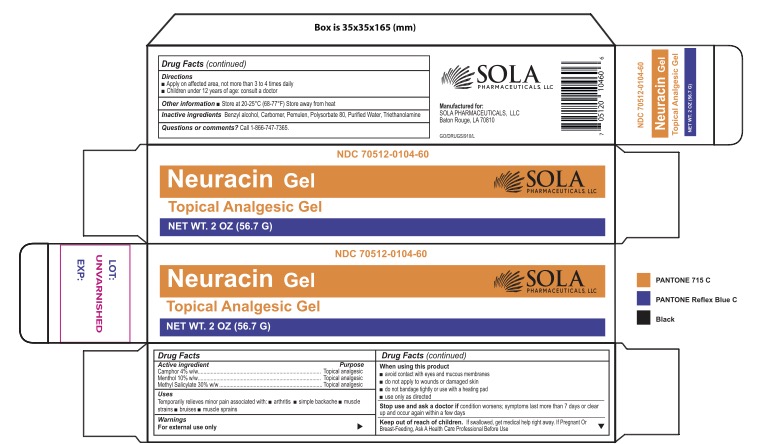
Label: NEURACIN TOPICAL GEL- neuracin topical analgesic gel gel
- NDC Code(s): 70512-104-60
- Packager: SOLA Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
For External use only
When using this product
- avoid contact with eyes and mucous membranes
- do no apply to wounds or damaged skin
- do not bandage tightly or use with a heating pad
- use only as directed
Stop use and ask a doctor if condition worsens; symptoms last more than 7 days or clear up and occur again within a few days
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEURACIN TOPICAL GEL
neuracin topical analgesic gel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70512-104 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 300 mg in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 40 mg in 1 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 100 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) TROLAMINE (UNII: 9O3K93S3TK) CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) CARBOMER 940 (UNII: 4Q93RCW27E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70512-104-60 1 in 1 CARTON 05/24/2021 1 56.7 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/24/2021 Labeler - SOLA Pharmaceuticals (080121345)