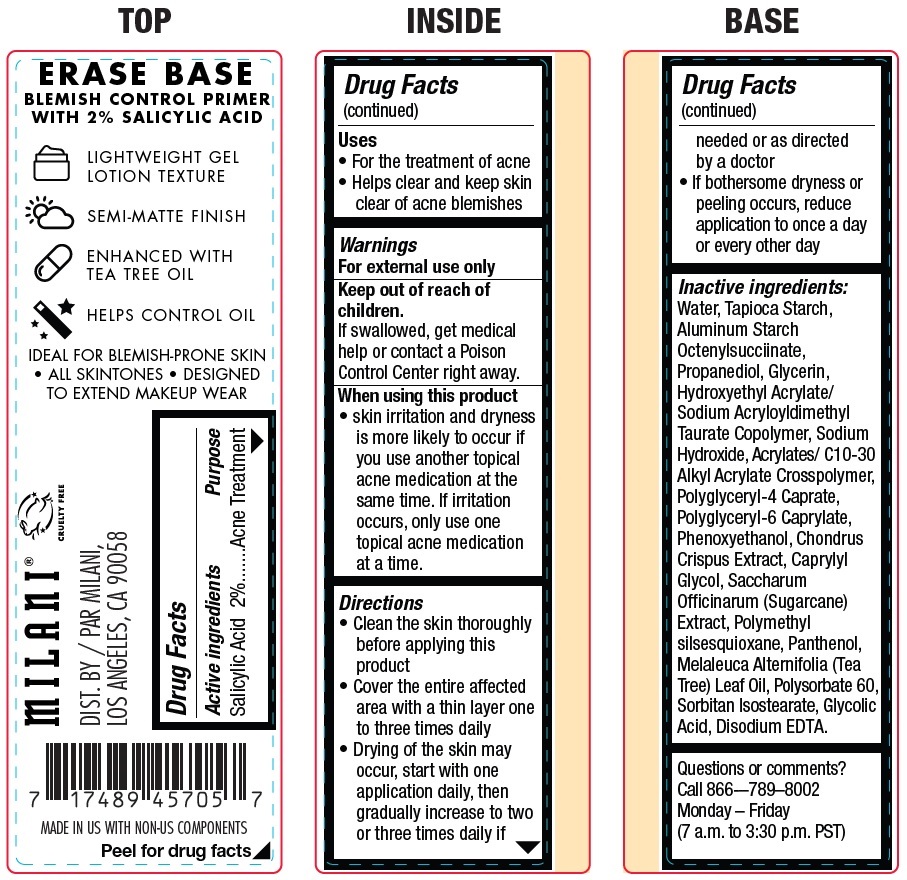
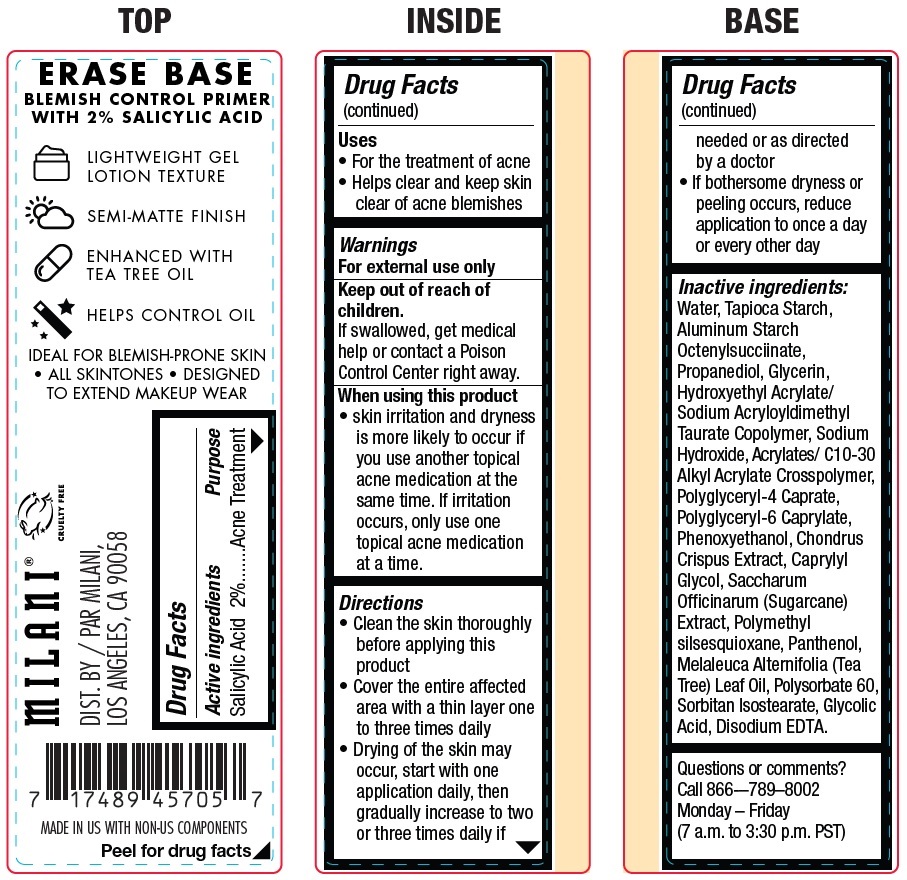
Label: MILANI ERASE BASE BLEMISH CONTROL PRIMER SALICYLIC ACID- salicylic acid cream
- NDC Code(s): 71435-0005-0
- Packager: New Milani Group, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
• Clean the skin thoroughly before applying this product • Cover the entire affected area with a thin layer one to three times daily • Drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor • If bothersome dryness or peeling occurs, reduce application to once a day or every other day
-
Inactive ingredients:
Water, Tapioca Starch, Aluminum Starch Octenylsucciinate, Propanediol, Glycerin, Hydroxyethyl Acrylate/ Sodium Acryloyldimethyl Taurate Copolymer, Sodium Hydroxide, Acrylates/ C10-30 Alkyl Acrylate Crosspolymer, Polyglyceryl-4 Caprate, Polyglyceryl-6 Caprylate, Phenoxyethanol, Chondrus Crispus Extract, Caprylyl Glycol, Saccharum Officinarum (Sugarcane) Extract, Polymethyl silsesquioxane, Panthenol, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Polysorbate 60, Sorbitan Isostearate, Glycolic Acid, Disodium EDTA.
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
MILANI ERASE BASE BLEMISH CONTROL PRIMER SALICYLIC ACID
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71435-0005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) STARCH, TAPIOCA (UNII: 24SC3U704I) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) SODIUM HYDROXIDE (UNII: 55X04QC32I) POLYGLYCERYL-4 CAPRATE (UNII: 3N873UN885) POLYGLYCERYL-6 CAPRYLATE (UNII: DGV8R54VG7) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHONDRUS CRISPUS CARRAGEENAN (UNII: UE856F2T78) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SUGARCANE (UNII: 81H2R5AOH3) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PANTHENOL (UNII: WV9CM0O67Z) TEA TREE OIL (UNII: VIF565UC2G) POLYSORBATE 60 (UNII: CAL22UVI4M) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) GLYCOLIC ACID (UNII: 0WT12SX38S) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71435-0005-0 30 mL in 1 TUBE; Type 0: Not a Combination Product 06/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/01/2022 Labeler - New Milani Group, LLC (120923342)