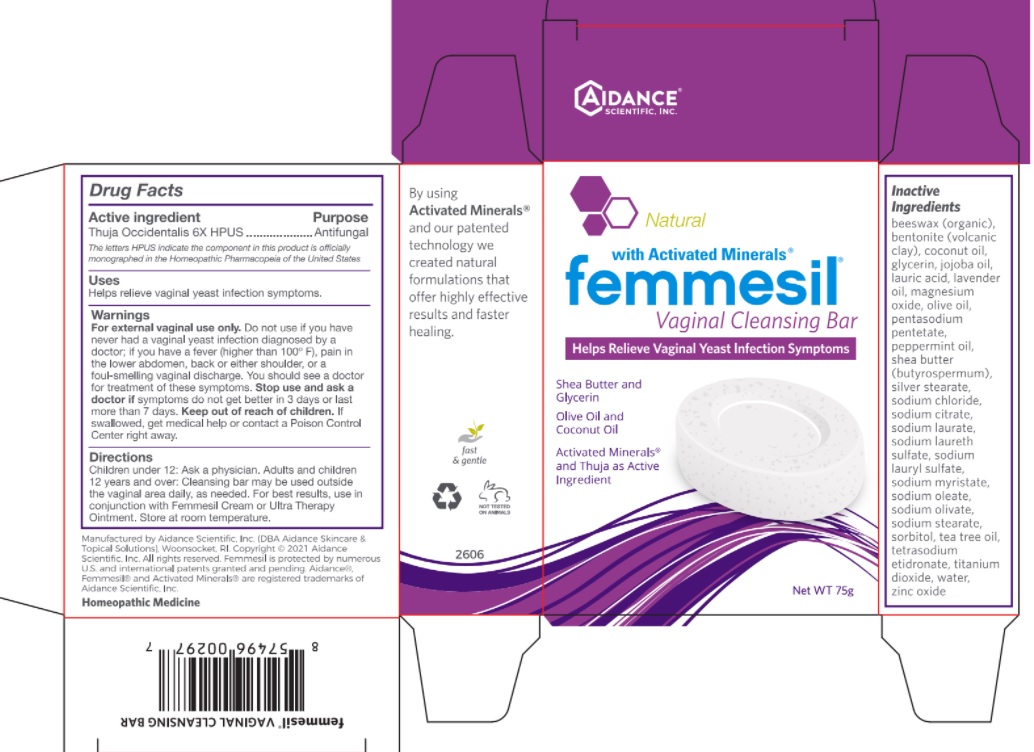
Label: FEMMESIL CLEANSING BAR- thuja occidentalis soap
- NDC Code(s): 24909-211-75
- Packager: Aidance Skincare & Topical Solutions, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 25, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external vaginal use only. Do not use if you have never had a vaginal yeast infection diagnosed by a doctor; if you have a fever (higher than 100° F), pain in the lower abdomen, back, or either shoulder, or a foul-smelling vaginal discharge. You should see a doctor for treatment of these symptoms.
Stop use and ask a doctor if symptoms do not get better in 3 days or last more than 7 days.
- Directions
-
Inactive Ingredients
beeswax (organic), bentonite (volcanic clay), coconut oil, glycerin, jojoba oil, lauric acid, lavender oil, magnesium oxide, olive oil, pentasodium pentetate, peppermint oil, shea butter (butyrospermum), silver stearate, sodium chloride, sodium citrate, sodium laurate, sodium laureth sulfate, sodium lauryl sulfate, sodium myristate, sodium oleate, sodium olivate, sodium stearate, sorbitol, tea tree oil, tetrasodium etidronate, titanium dioxide, water, zinc oxide
- Questions?
- Product label
-
INGREDIENTS AND APPEARANCE
FEMMESIL CLEANSING BAR
thuja occidentalis soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24909-211 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THUJA OCCIDENTALIS LEAF (UNII: 0T0DQN8786) (THUJA OCCIDENTALIS LEAF - UNII:0T0DQN8786) THUJA OCCIDENTALIS LEAF 6 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) BENTONITE (UNII: A3N5ZCN45C) COCONUT OIL (UNII: Q9L0O73W7L) GLYCERIN (UNII: PDC6A3C0OX) JOJOBA OIL (UNII: 724GKU717M) LAURIC ACID (UNII: 1160N9NU9U) LAVENDER OIL (UNII: ZBP1YXW0H8) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) OLIVE OIL (UNII: 6UYK2W1W1E) PENTASODIUM PENTETATE (UNII: 961TOZ5L7T) PEPPERMINT OIL (UNII: AV092KU4JH) SHEA BUTTER (UNII: K49155WL9Y) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM LAURATE (UNII: K146MR5EXO) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM MYRISTATE (UNII: 06BLC4V0IV) SODIUM OLEATE (UNII: 399SL044HN) SODIUM STEARATE (UNII: QU7E2XA9TG) SORBITOL (UNII: 506T60A25R) TEA TREE OIL (UNII: VIF565UC2G) ETIDRONATE TETRASODIUM (UNII: CZZ9T1T1X4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) ZINC OXIDE (UNII: SOI2LOH54Z) SILVER OXIDE (UNII: 897WUN6G6T) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM OLIVATE (UNII: ND5Y5M6ZUT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24909-211-75 75 g in 1 BOX; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/30/2022 Labeler - Aidance Skincare & Topical Solutions, LLC (018950611) Establishment Name Address ID/FEI Business Operations Aidance Skincare & Topical Solutions, LLC 018950611 manufacture(24909-211)