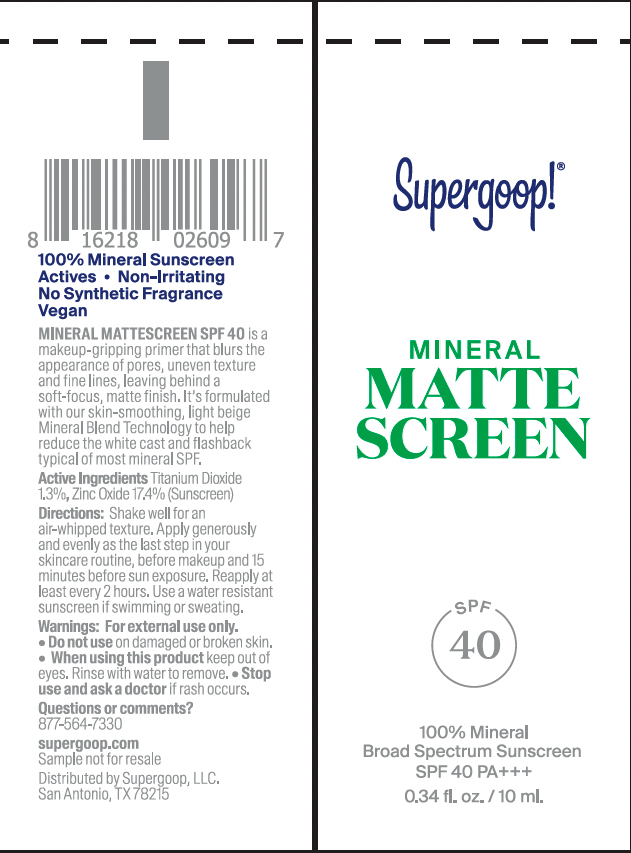
Label: MINERAL MATTESCREEN SPF 40- titanium dioxide, zinc oxide cream
-
NDC Code(s):
75936-281-01,
75936-281-02,
75936-281-03,
75936-281-04, view more75936-281-05, 75936-281-06
- Packager: Supergoop, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
- Apply generously and evenly 15 minutes before sun exposure
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating
- Sun Protection Measures Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use sunscreen with a Broad-Spectrum SPF value of 15 or higher and other sunprotection measures including: • limit your time in the sun, especially from 10a.m. – 2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: ask a doctor.
-
INACTIVE INGREDIENT
Inactive Ingredients
Dimethicone, Dimethicone Copolymer, Caprylic/Capric Triglyceride, Methyl Dihydroabietate, Polyhydroxystearic Acid, C9-12 Alkane, Glycerin, Iron Oxides (CI 77492), Ethylhexylglycerin, Water (Aqua), Coco-Caprylate/Caprate, Silica, Stearic Acid, Iron Oxides (CI 77491), Iron Oxides (CI 77499), Hedychium Coronarium Root Extract, Lactobacillus/Arundinaria Gigantea Leaf Ferment Filtrate, Leuconostoc/Radish Root Ferment Filtrate, Titanium Dioxide (CI 77891)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MINERAL MATTESCREEN SPF 40
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-281 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.3 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 17.4 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHYL DIHYDROABIETATE (UNII: 7666FJ0J9F) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) STEARIC ACID (UNII: 4ELV7Z65AP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HEDYCHIUM CORONARIUM ROOT (UNII: 92A6N0IQN9) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-281-01 5 mL in 1 TUBE; Type 0: Not a Combination Product 05/18/2021 2 NDC:75936-281-02 10 mL in 1 TUBE; Type 0: Not a Combination Product 05/18/2021 3 NDC:75936-281-03 45 mL in 1 CARTON; Type 0: Not a Combination Product 05/18/2021 4 NDC:75936-281-04 20 mL in 1 TUBE; Type 0: Not a Combination Product 09/02/2021 5 NDC:75936-281-05 1.5 mL in 1 PACKET; Type 0: Not a Combination Product 05/18/2021 6 NDC:75936-281-06 15 mL in 1 TUBE; Type 0: Not a Combination Product 05/18/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/18/2021 Labeler - Supergoop, LLC (117061743)