Label: AVENE SOLAIRE UV MINERAL SUNSCREEN SPF 50 PLUS- zinc oxide lotion
- NDC Code(s): 64760-724-01
- Packager: Pierre Fabre USA Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
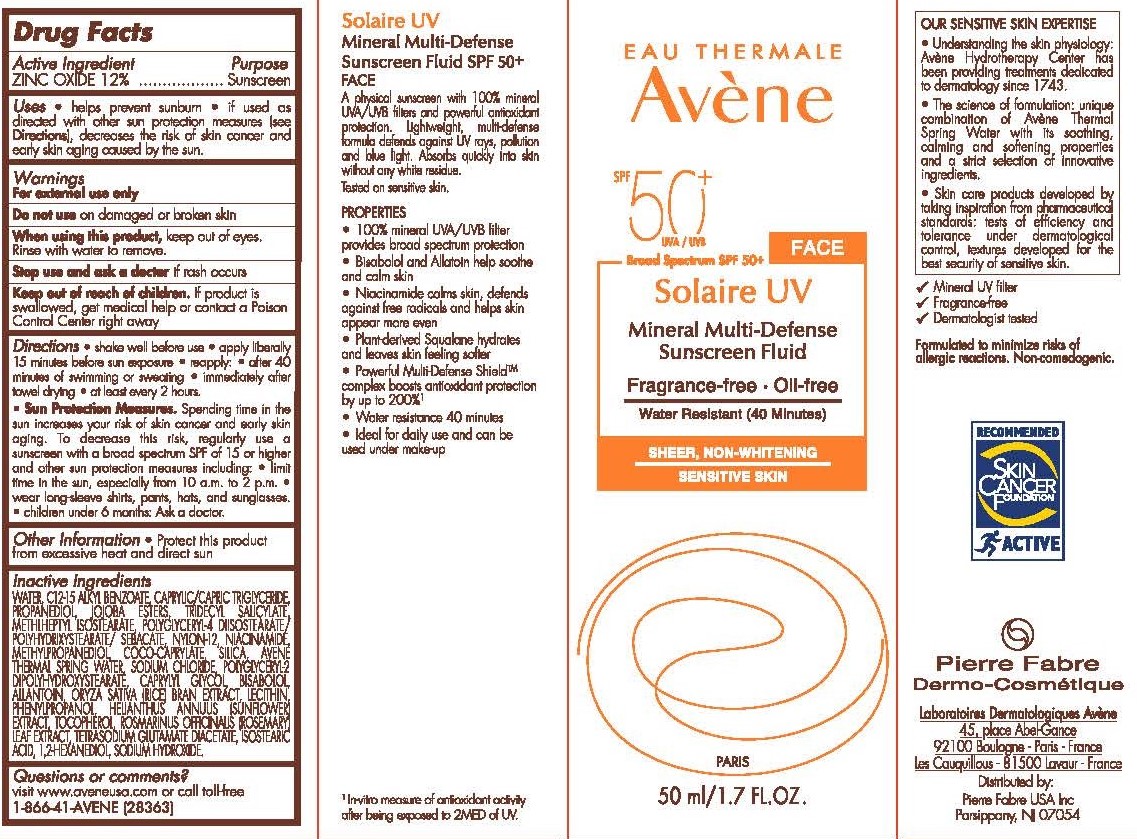
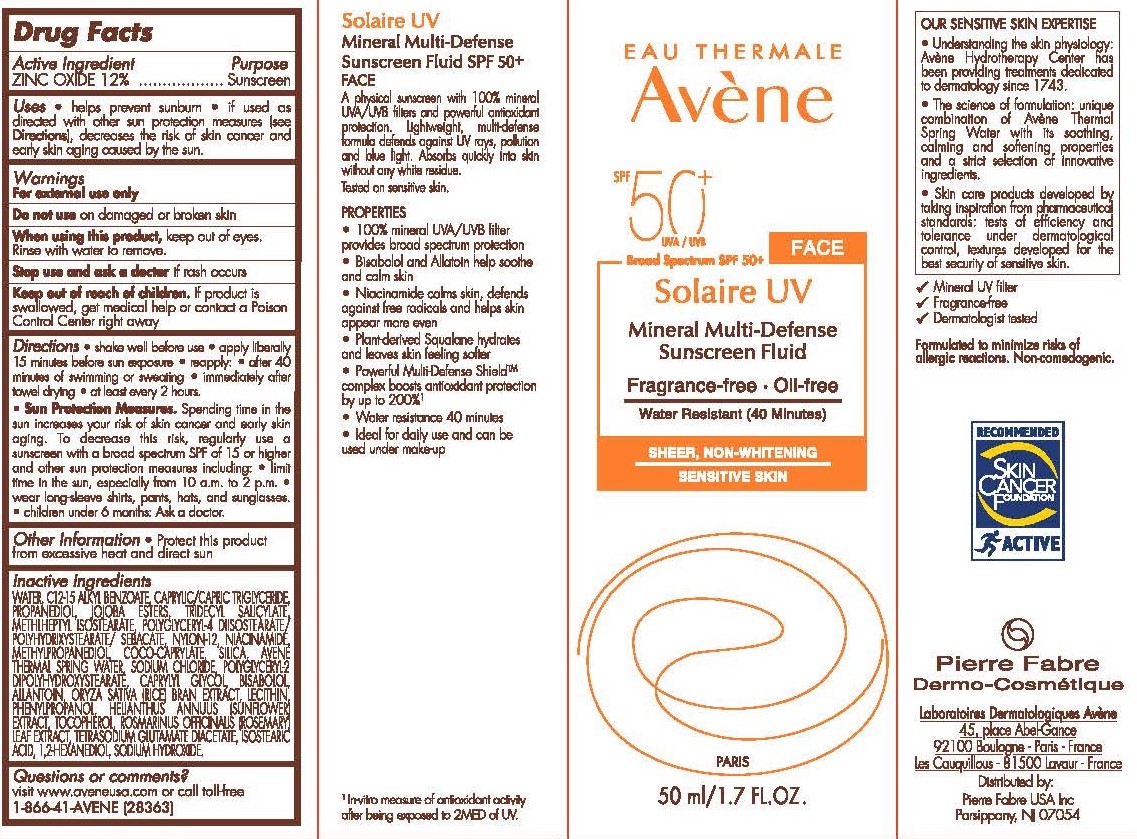
- Drug Facts
- Purpose
- Active Ingredient
- Uses
- Warnings
-
Directions
Shake well before use.
Apply liberally 15 minutes before sun exposure.
Reapply.
After 80 minutes of swimming or sweating.
Immediately after towel dry.
At least every 2 hours.
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk of skin cancer and early skin gaining. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
Limit time in the sun, especially from 10 a.m to 2 p.m.
Wear long-sleeve shirts, pants, hats, and sunglasses.
Children under 6 months: Ask a doctor.
- Other information
-
Inactive ingredients
AVÈNE THERMAL SPRING WATER, C12-15 ALKYL BENZOATE,
CAPRYLIC/CAPRIC TRIGLYCERIDE, PROPANEDIOL, JOJOBA ESTERS,
TRIDECYL SALICYLATE, METHYLHEPTYL ISOSTEARATE, POLYGLYCERYL-4
DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE, NYLON-12,
NIACINAMIDE, METHYLPROPANEDIOL, COCO-CAPRYLATE, SILICA,
SODIUM CHLORIDE, POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE,
CAPRYLYL GLYCOL, BISABOLOL, ALLANTOIN, ORYZA SATIVA (RICE)
BRAN EXTRACT, LECITHIN, PHENYLPROPANOL, HELIANTHUS
ANNUUS (SUNFLOWER) EXTRACT, TOCOPHEROL, ROSMARINUS
OFFICINALIS (ROSEMARY) LEAF EXTRACT, TETRASODIUM
GLUTAMATE DIACETATE, ISOSTEARIC ACID, 1,2-HEXANEDIOL,
SODIUM HYDROXIDE - Questions or comments?
- Principal Display Panel - 50 mL Carton
-
INGREDIENTS AND APPEARANCE
AVENE SOLAIRE UV MINERAL SUNSCREEN SPF 50 PLUS
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64760-724 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength ROSEMARY OIL (UNII: 8LGU7VM393) LEVOMENOL (UNII: 24WE03BX2T) TRIDECYL SALICYLATE (UNII: AZQ08K38Z1) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) NIACINAMIDE (UNII: 25X51I8RD4) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPANEDIOL (UNII: 5965N8W85T) SODIUM CHLORIDE (UNII: 451W47IQ8X) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISOSTEARIC ACID (UNII: X33R8U0062) SODIUM HYDROXIDE (UNII: 55X04QC32I) JOJOBA OIL (UNII: 724GKU717M) METHYLHEPTYL ISOSTEARATE (UNII: 981F40Q9FF) NYLON-12 (UNII: 446U8J075B) METHYLPROPANEDIOL (UNII: N8F53B3R4R) COCO-CAPRYLATE (UNII: 4828G836N6) WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ALLANTOIN (UNII: 344S277G0Z) RICE BRAN OIL (UNII: LZO6K1506A) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) PHENYLPROPANOL (UNII: 0F897O3O4M) HELIANTHUS ANNUUS WHOLE (UNII: 17S27ZT6KR) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64760-724-01 1 in 1 CARTON 05/17/2021 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/17/2021 11/06/2025 Labeler - Pierre Fabre USA Inc. (117196928) Registrant - Pierre Fabre USA Inc. (117196928)