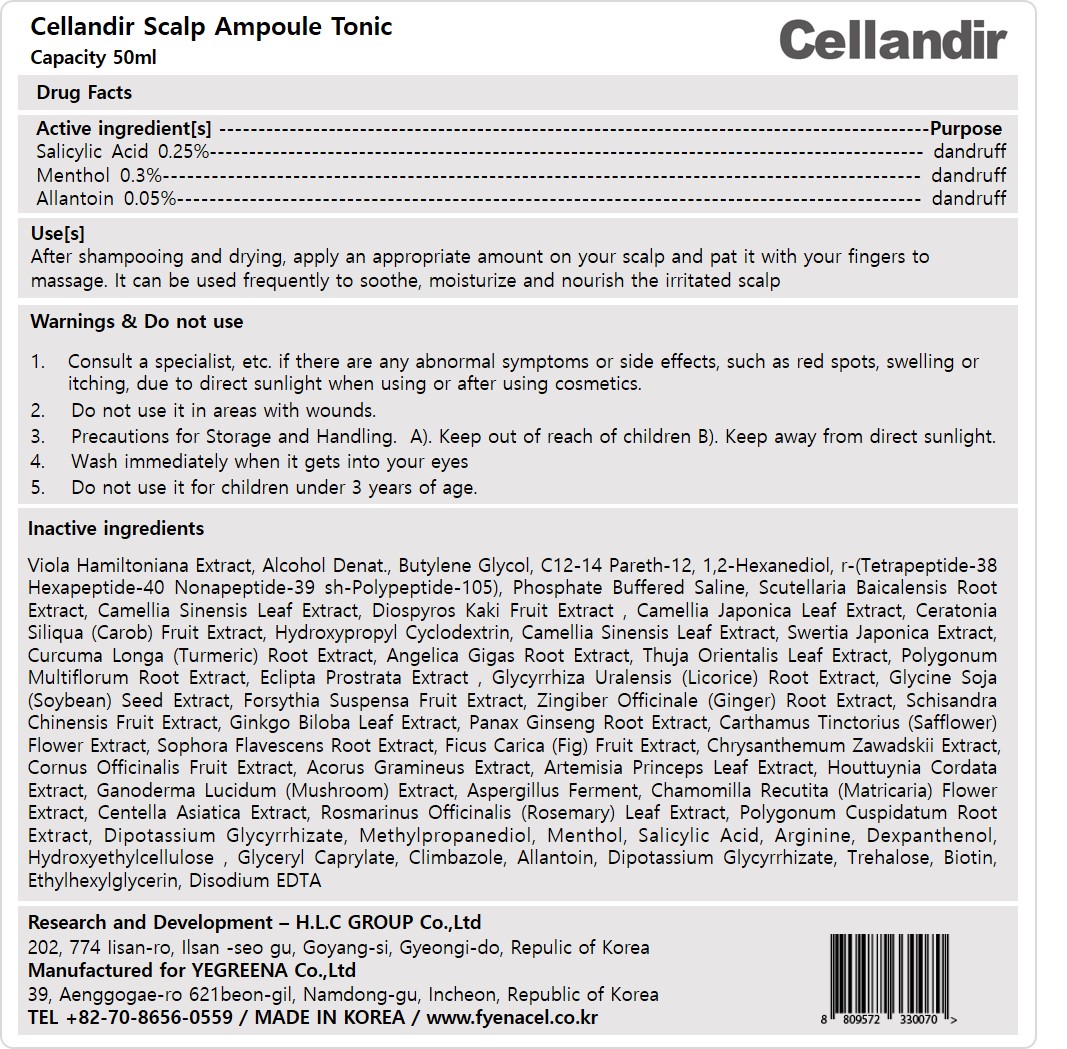
Label: CELLANDIR SCALP AMPOULE- menthol, salicylic acid, allantoin liquid
- NDC Code(s): 81555-202-01
- Packager: H.L.C GROUP Co., ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient[s]
- Purpose
- Use[s]
- Warnings
- Do not use
- Stop use and ask a doctor
- Keep out of reach of children
- Directions
-
Inactive ingredients
Viola Hamiltoniana Extract, Alcohol Denat., Butylene Glycol, C12-14 Pareth-12, 1,2-Hexanediol, r-(Tetrapeptide-38 Hexapeptide-40 Nonapeptide-39 sh-Polypeptide-105), Phosphate Buffered Saline, Scutellaria Baicalensis Root Extract, Camellia Sinensis Leaf Extract, Diospyros Kaki Fruit Extract , Camellia Japonica Leaf Extract, Ceratonia Siliqua (Carob) Fruit Extract, Hydroxypropyl Cyclodextrin, Camellia Sinensis Leaf Extract, Swertia Japonica Extract, Curcuma Longa (Turmeric) Root Extract, Angelica Gigas Root Extract, Thuja Orientalis Leaf Extract, Polygonum Multiflorum Root Extract, Eclipta Prostrata Extract , Glycyrrhiza Uralensis (Licorice) Root Extract, Glycine Soja (Soybean) Seed Extract, Forsythia Suspensa Fruit Extract, Zingiber Officinale (Ginger) Root Extract, Schisandra Chinensis Fruit Extract, Ginkgo Biloba Leaf Extract, Panax Ginseng Root Extract, Carthamus Tinctorius (Safflower) Flower Extract, Sophora Flavescens Root Extract, Ficus Carica (Fig) Fruit Extract, Chrysanthemum Zawadskii Extract, Cornus Officinalis Fruit Extract, Acorus Gramineus Extract, Artemisia Princeps Leaf Extract, Houttuynia Cordata Extract, Ganoderma Lucidum (Mushroom) Extract, Aspergillus Ferment, Chamomilla Recutita (Matricaria) Flower Extract, Centella Asiatica Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Polygonum Cuspidatum Root Extract, Dipotassium Glycyrrhizate, Methylpropanediol, Menthol, Salicylic Acid, Arginine, Dexpanthenol, Hydroxyethylcellulose , Glyceryl Caprylate, Climbazole, Allantoin, Dipotassium Glycyrrhizate, Trehalose, Biotin, Ethylhexylglycerin, Disodium EDTA
- Package Label
-
INGREDIENTS AND APPEARANCE
CELLANDIR SCALP AMPOULE
menthol, salicylic acid, allantoin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81555-202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 0.3 mg in 100 mL SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.25 mg in 100 mL ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 0.05 mg in 100 mL Inactive Ingredients Ingredient Name Strength CAMELLIA JAPONICA LEAF (UNII: 4E3VE6KTLY) TURMERIC (UNII: 856YO1Z64F) PLATYCLADUS ORIENTALIS LEAF (UNII: 32E5V7G32B) ECLIPTA PROSTRATA LEAF (UNII: H86R96580E) GLYCYRRHIZA URALENSIS ROOT (UNII: 42B5YD8F0K) GINGER (UNII: C5529G5JPQ) SAFFLOWER (UNII: 4VBL71TY4Y) ACORUS GRAMINEUS ROOT (UNII: Z60N6Q6E19) ARTEMISIA PRINCEPS LEAF (UNII: SY077EW02G) CENTELLA ASIATICA WHOLE (UNII: 7M867G6T1U) REYNOUTRIA JAPONICA ROOT (UNII: 7TRV45YZF7) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) GLYCERYL MONOCAPRYLATE (UNII: TM2TZD4G4A) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ALCOHOL (UNII: 3K9958V90M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) SOYBEAN (UNII: L7HT8F1ZOD) ROSEMARY (UNII: IJ67X351P9) BIOTIN (UNII: 6SO6U10H04) TREHALOSE (UNII: B8WCK70T7I) C12-14 PARETH-12 (UNII: M0LJS773XW) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) REISHI (UNII: TKD8LH0X2Z) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) SWERTIA JAPONICA WHOLE FLOWERING (UNII: 01X0P6GX6C) ANGELICA GIGAS ROOT (UNII: 32766B2FHX) GINKGO (UNII: 19FUJ2C58T) ARGININE (UNII: 94ZLA3W45F) PANTHENOL (UNII: WV9CM0O67Z) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) REYNOUTRIA MULTIFLORA ROOT (UNII: AUZ3VD75MC) PERSIMMON (UNII: 4V023DD7KL) CAROB (UNII: 5MG5Z946UO) HYDROXYPROPYL BETADEX (UNII: 1I96OHX6EK) FORSYTHIA SUSPENSA FRUIT (UNII: P4793M1ES5) CORNUS OFFICINALIS FRUIT (UNII: 23NL8NQ187) CHRYSANTHEMUM ZAWADSKII WHOLE (UNII: 8Z1G3DJ591) CHAMOMILE (UNII: FGL3685T2X) SCHISANDRA CHINENSIS FRUIT (UNII: ABS794681C) ASIAN GINSENG (UNII: CUQ3A77YXI) FIG (UNII: TGD87RII2U) CLIMBAZOLE (UNII: 9N42CW7I54) GREEN TEA LEAF (UNII: W2ZU1RY8B0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81555-202-01 50 mL in 1 AMPULE; Type 0: Not a Combination Product 05/06/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 05/06/2021 Labeler - H.L.C GROUP Co., ltd (694869128) Registrant - H.L.C GROUP Co., ltd (694869128) Establishment Name Address ID/FEI Business Operations H.L.C GROUP Co., ltd 695436080 manufacture(81555-202)