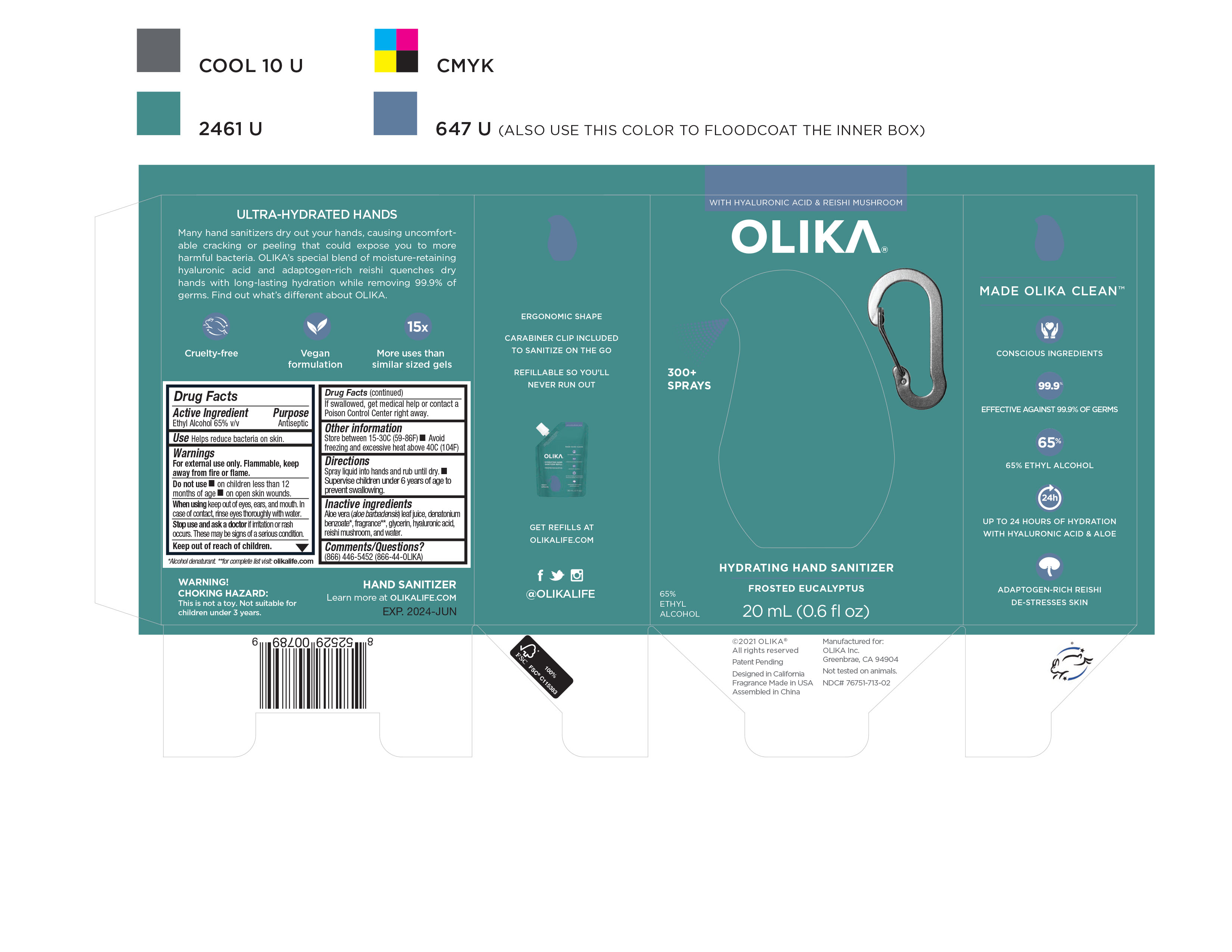
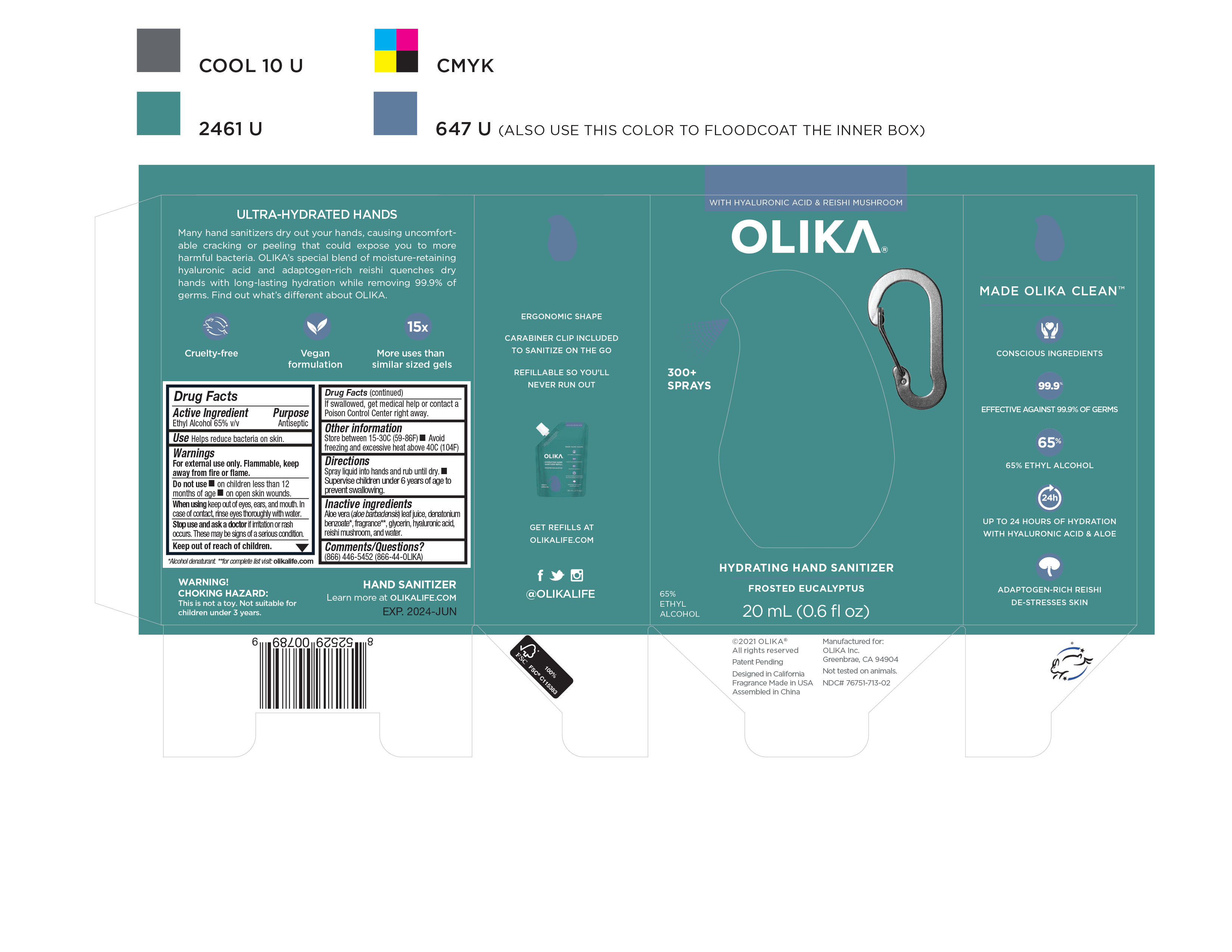
Label: OLIKA HYDRATING HAND SANITIZER FROSTED EUCALYPTUS- alcohol liquid
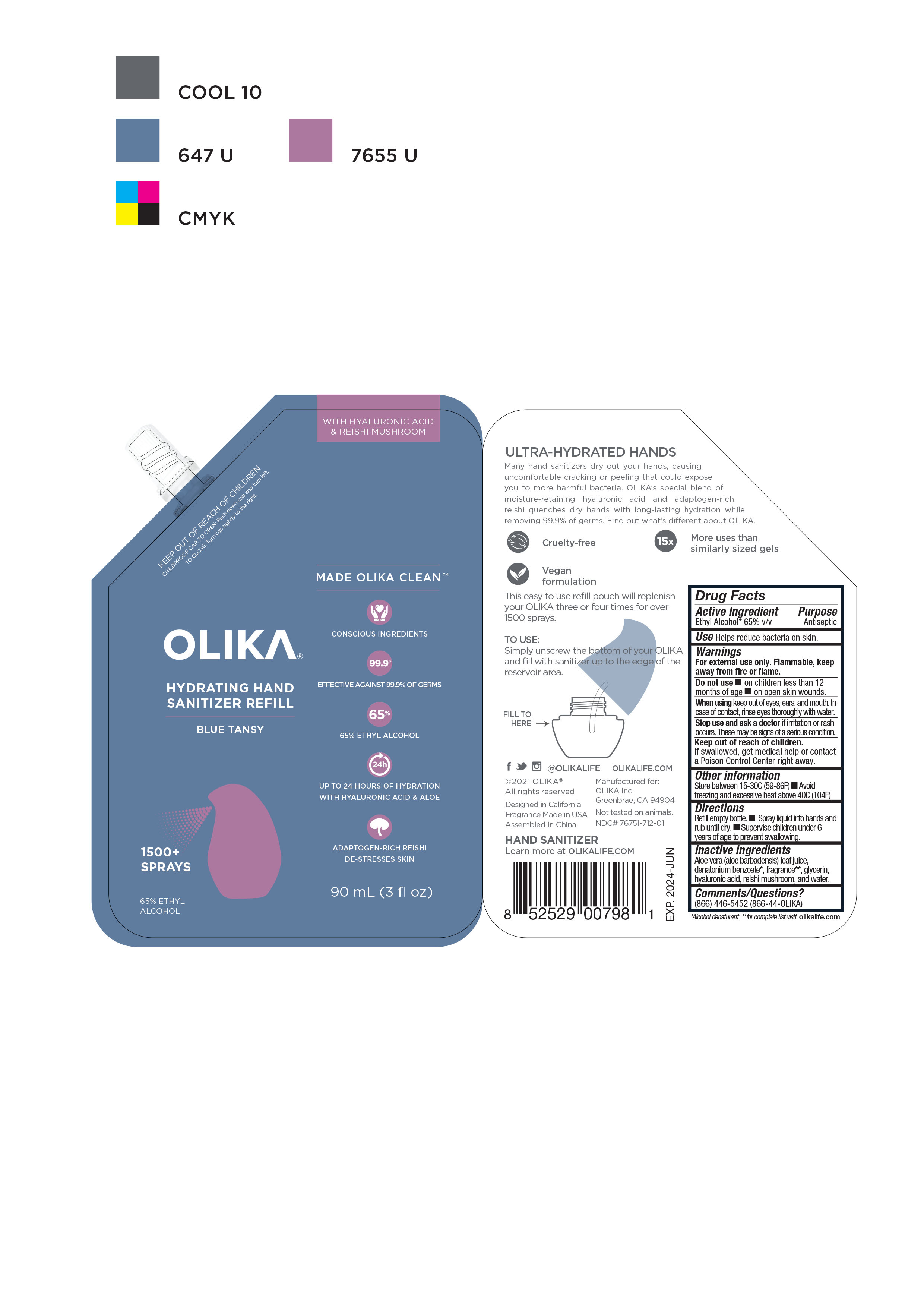
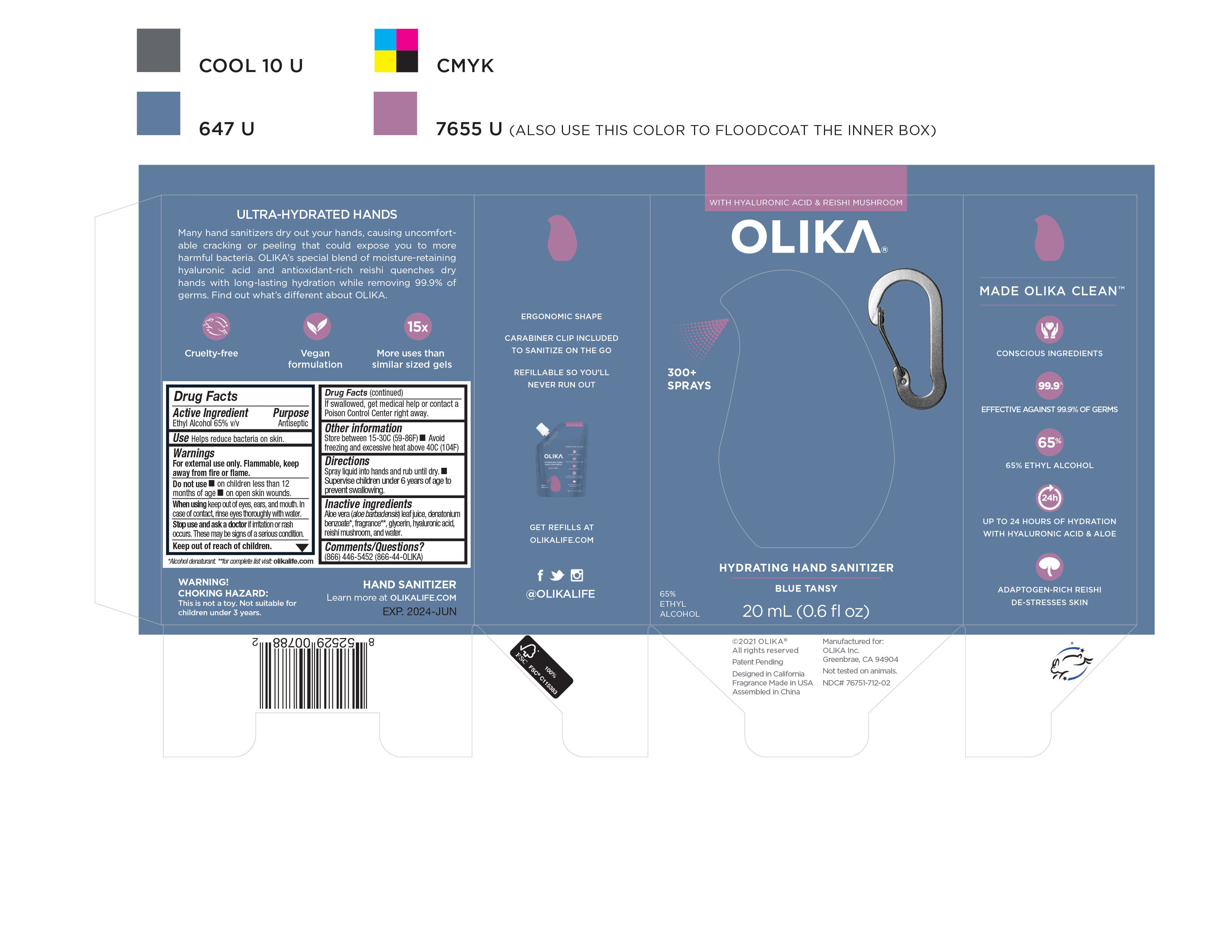
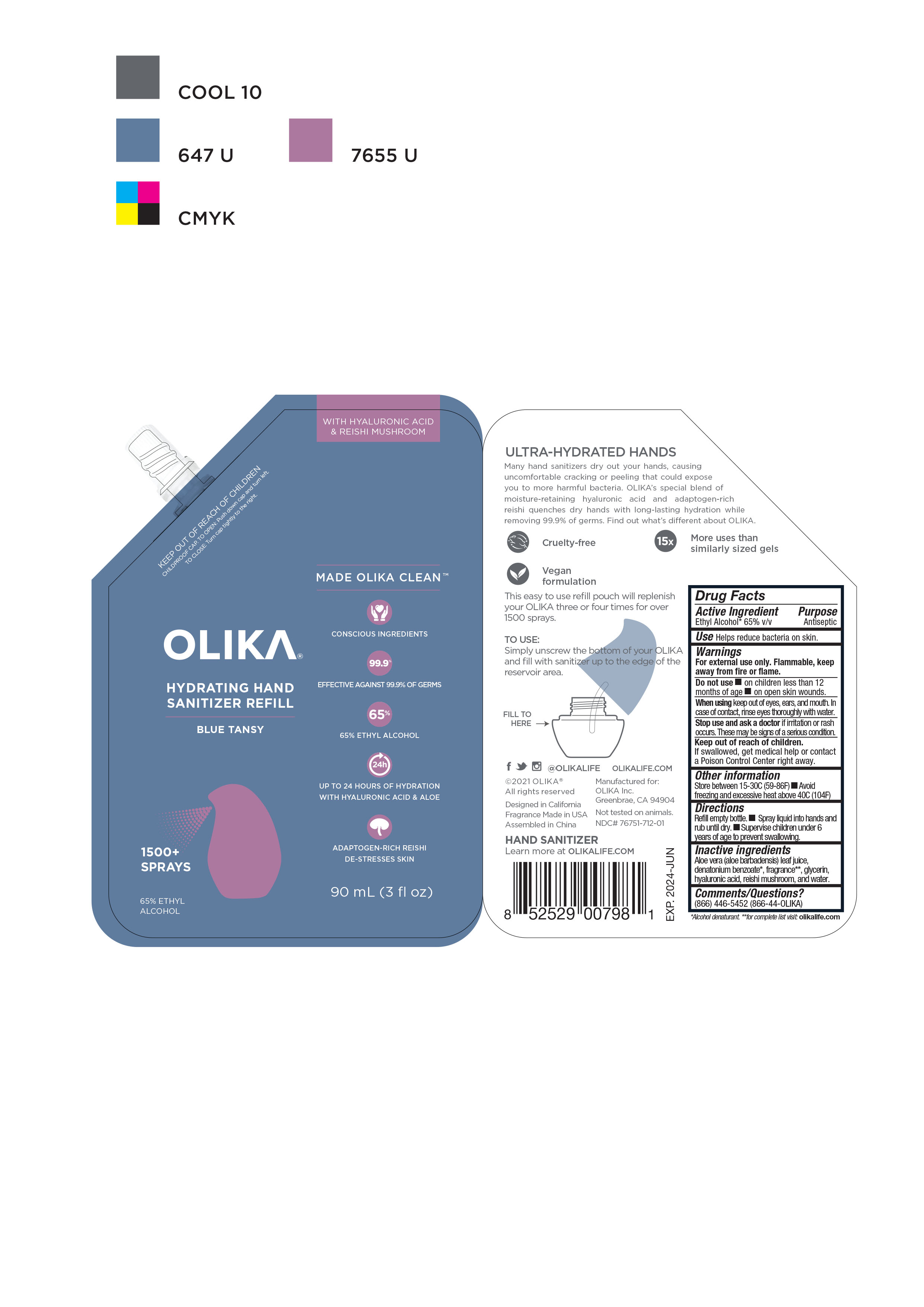
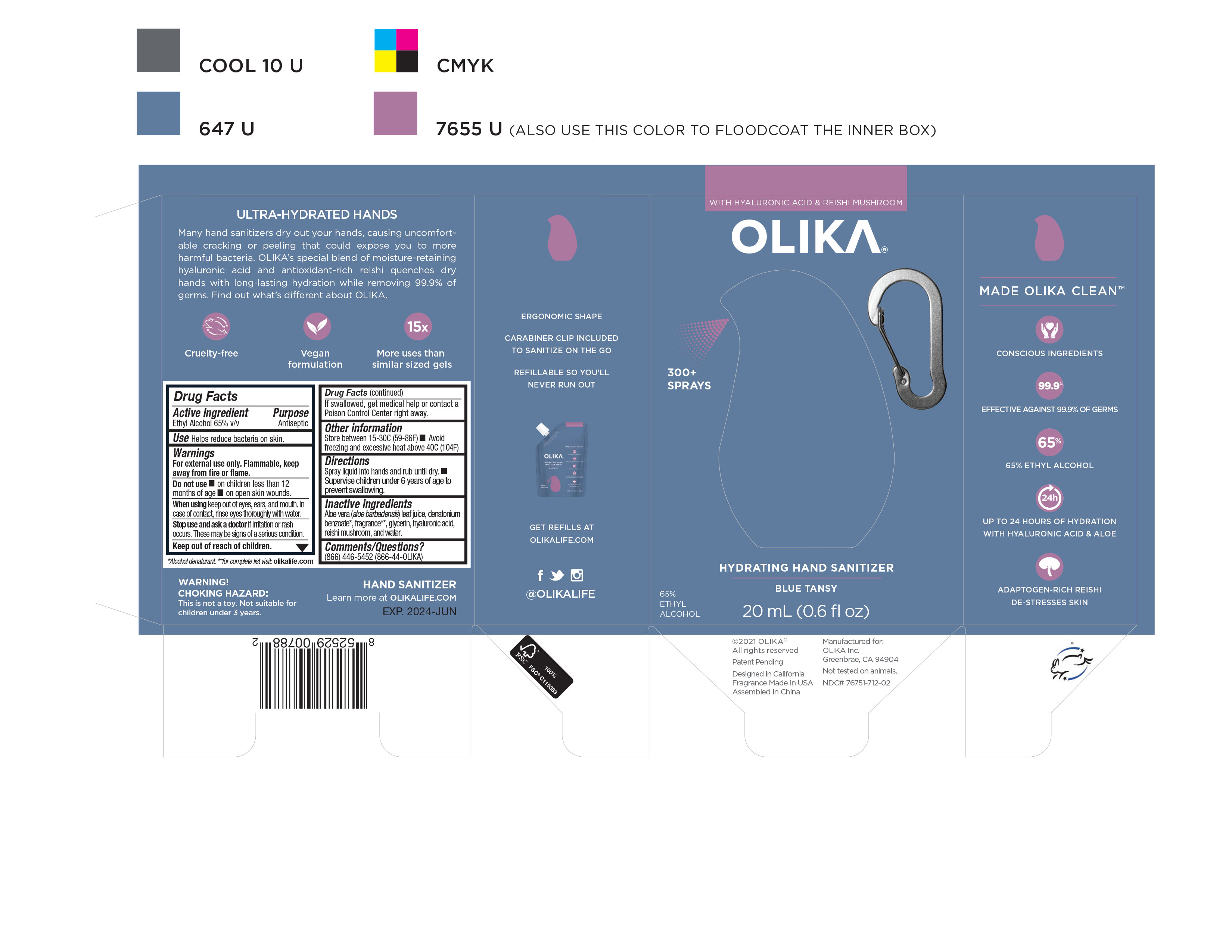
OLIKA HYDRATING HAND SANITIZER BLUE TANSY- alcohol liquid
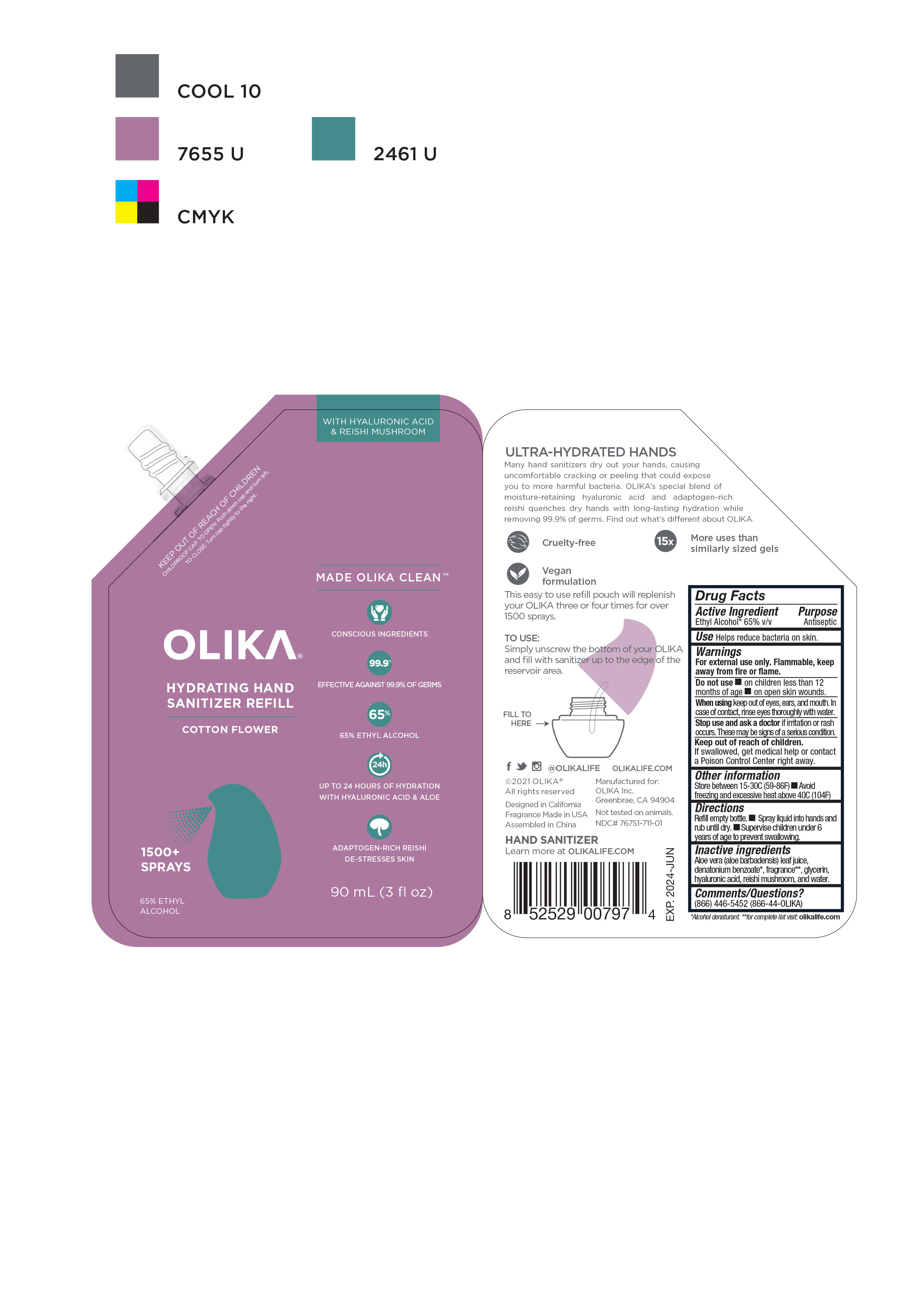
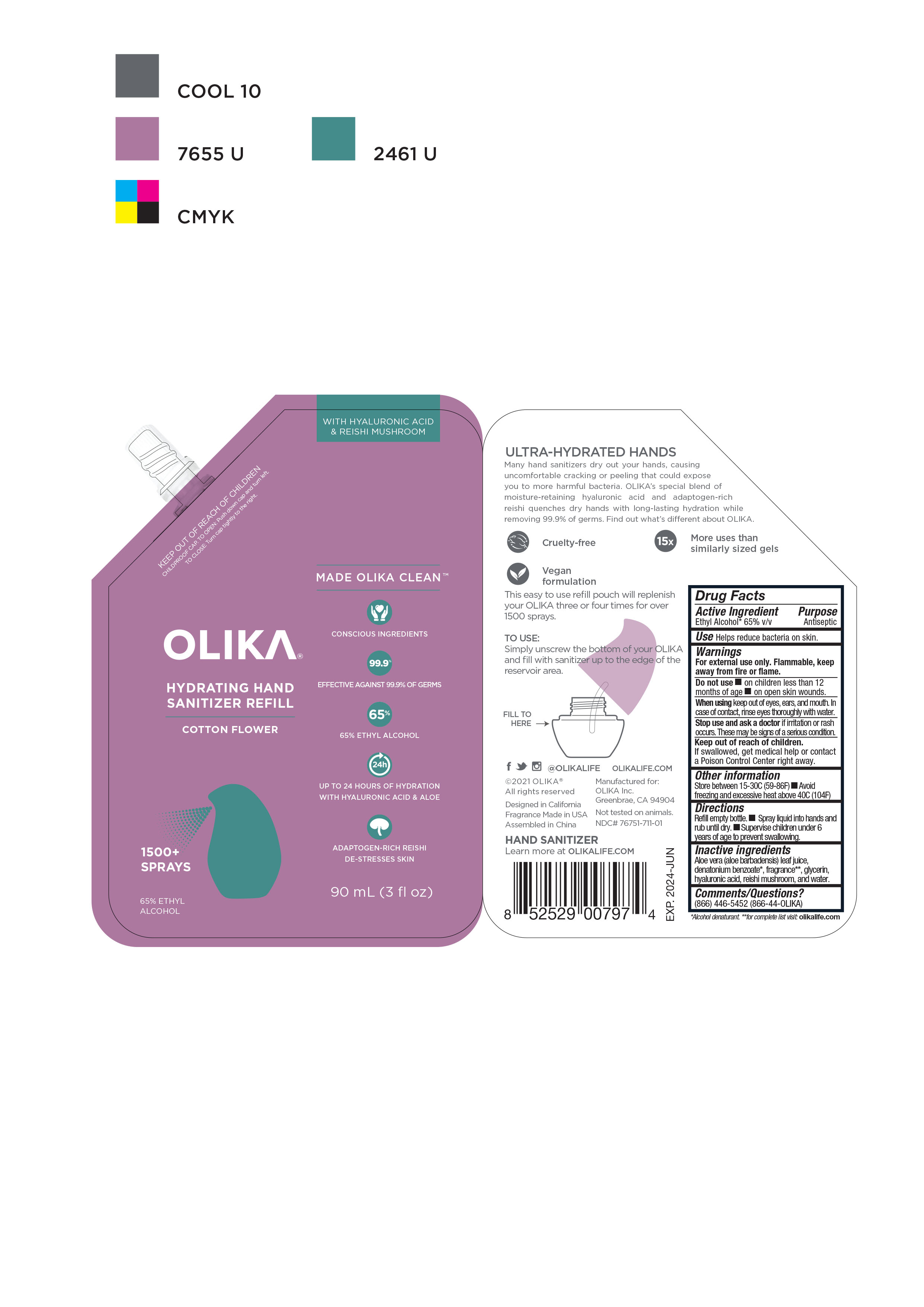
OLIKA HYDRATING HAND SANITIZER COTTON FLOWER- alcohol liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 76751-711-01, 76751-711-02, 76751-711-20, 76751-712-01, view more76751-712-02, 76751-712-20, 76751-713-00, 76751-713-01, 76751-713-02 - Packager: Olika Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 10, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- 20 mL Primary Container Spray Bottle Bottom Label
- Package Label - Principal Display Panel and Information Panels
- Package Label - Principal Display Panel and Information Panels
- Package Label - Principal Display Panel and Information Panels
- Package Label - Principal Display Panel and Information Panels
- Package Label - Principal Display Panel and Information Panels
- Package Label - Principal Display Panel and Information Panels
-
INGREDIENTS AND APPEARANCE
OLIKA HYDRATING HAND SANITIZER FROSTED EUCALYPTUS
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76751-713 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65 mL in 100 mL Inactive Ingredients Ingredient Name Strength ACETYL CEDRENE (UNII: X6I62755AK) 0.01667 mL in 100 mL IONONE (UNII: QP734LIN1K) 0.00667 mL in 100 mL ETHYL LINALOOL (UNII: SF2JS9GF5T) 0.005 mL in 100 mL ETHYL BUTYRATE (UNII: UFD2LZ005D) 0.005 mL in 100 mL FLORALOZONE (UNII: 1HA71K8K9L) 0.00417 mL in 100 mL METHYL BENZODIOXEPINONE (UNII: 0NQ136C313) 0.00167 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 4.2 mL in 100 mL WATER (UNII: 059QF0KO0R) 29.78 mL in 100 mL NERYL ACETATE (UNII: OF82IJU18H) 0.00833 mL in 100 mL DIPROPYLENE GLYCOL (UNII: E107L85C40) 0.14825 mL in 100 mL 2-ISOBUTYL-4-METHYLTETRAHYDROPYRAN-4-OL (UNII: VK5ZHH2T3F) 0.08333 mL in 100 mL ALLYL HEPTANOATE (UNII: AU4CYG9V68) 0.00033 mL in 100 mL METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) 0.075 mL in 100 mL DIHYDROMYRCENOL (UNII: 46L1B02ND9) 0.03333 mL in 100 mL ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) 0.06667 mL in 100 mL 3-ISOCAMPHYLCYCLOHEXANOL, TRANS- (UNII: 34UP96K73Z) 0.025 mL in 100 mL HEXYL ACETATE (UNII: 7U7KU3MWT0) 0.00667 mL in 100 mL GERANYL ACETATE (UNII: 3W81YG7P9R) 0.005 mL in 100 mL ETHYL ACETOACETATE ETHYLENEGLYCOL KETAL (UNII: G5EXI4NID0) 0.00167 mL in 100 mL PENTADECALACTONE (UNII: OK17S3S98K) 0.00167 mL in 100 mL CYCLOPENTANONE (UNII: 220W81TN3S) 0.00167 mL in 100 mL ETHYL 2,4-DECADIENOATE, (2E,4Z)- (UNII: 79P6KS9Y5Z) 0.00083 mL in 100 mL 4-(P-HYDROXYPHENYL)-2-BUTANONE (UNII: 7QY1MH15BG) 0.00083 mL in 100 mL HYALURONATE SODIUM (UNII: YSE9PPT4TH) 0.07 mL in 100 mL ALLYL .ALPHA.-IONONE (UNII: 8IP66F9ODG) 0.00083 mL in 100 mL ALLYL HEXANOATE (UNII: 3VH84A363D) 0.00083 mL in 100 mL ALLYL CYCLOHEXANEACETATE (UNII: M6J8835739) 0.00058 mL in 100 mL 3-HEXEN-1-OL, (3Z)- (UNII: V14F8G75P4) 0.0005 mL in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.25 mL in 100 mL REISHI (UNII: TKD8LH0X2Z) 0.2 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76751-713-02 1 in 1 BLISTER PACK 06/14/2021 1 NDC:76751-713-00 20 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 2 NDC:76751-713-01 90 mL in 1 POUCH; Type 0: Not a Combination Product 06/14/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/14/2021 OLIKA HYDRATING HAND SANITIZER BLUE TANSY
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76751-712 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65 mL in 100 mL Inactive Ingredients Ingredient Name Strength ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) 0.1021 mL in 100 mL TETRAMETHYL ACETYLOCTAHYDRONAPHTHALENES (UNII: 2JU6ZH6GRE) 0.0592 mL in 100 mL DIPROPYLENE GLYCOL (UNII: E107L85C40) 0.1567 mL in 100 mL ETHYL 2-METHYLBUTYRATE (UNII: L1T4AB29DS) 0.00002 mL in 100 mL METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) 0.0828 mL in 100 mL 2-ISOBUTYL-4-METHYLTETRAHYDROPYRAN-4-OL (UNII: VK5ZHH2T3F) 0.0237 mL in 100 mL CYCLODODECANE (UNII: 97CN13ZD83) 0.0207 mL in 100 mL 2-TERT-BUTYLCYCLOHEXYL ACETATE (UNII: 364FV60913) 0.008 mL in 100 mL NAPHTHO(2,1-B)FURAN (UNII: 5O098D6W4T) 0.0033 mL in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.25 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 4.2 mL in 100 mL WATER (UNII: 059QF0KO0R) 29.78 mL in 100 mL 4-HYDROXY-1-(5-ISOCAMPHYL)CYCLOHEXANE (UNII: TVJ25725XP) 0.0142 mL in 100 mL DIETHYL MALONATE (UNII: 53A58PA183) 0.0104 mL in 100 mL 2,4-DIMETHYL-4-NONANOL (UNII: 625246Q74Q) 0.0053 mL in 100 mL 4-TERT-BUTYLCYCLOHEXYL ACETATE (UNII: 21EUM2B8UC) 0.0044 mL in 100 mL 3-ISOCAMPHYLCYCLOHEXANOL, TRANS- (UNII: 34UP96K73Z) 0.0036 mL in 100 mL CEDRYL ACETATE (UNII: 0WS0WJ9WNV) 0.0015 mL in 100 mL AMBROXIDE, (-)- (UNII: TD34B3O8M9) 0.0009 mL in 100 mL METHYL 3-METHYLORSELLINATE (UNII: 12YH9T04QE) 0.0006 mL in 100 mL 4-METHYL-3-DECEN-5-OL, (3E)- (UNII: 5S5I61TY7P) 0.0004 mL in 100 mL DIHYDRODEHYDRO-.BETA.-IONONE (UNII: XZS4VD987O) 0.0002 mL in 100 mL ETHYL 2-METHYLPENTANOATE (UNII: 405SN8638D) 0.0002 mL in 100 mL .GAMMA.-DECALACTONE (UNII: 7HLS05KP9O) 0.0001 mL in 100 mL DECAHYDRO-2,2,6,6,7,8,8-HEPTAMETHYL-2H-INDENO(4,5-B)FURAN (UNII: 89ZYZ7YI66) 0.00004 mL in 100 mL METHYL ANTHRANILATE (UNII: 981I0C1E5W) 0.00002 mL in 100 mL 2,4-DIMETHYL-3-CYCLOHEXENE CARBOXALDEHYDE (UNII: 452GFV2AFS) 0.0002 mL in 100 mL 3-HEXEN-1-OL, (3Z)- (UNII: V14F8G75P4) 0.0004 mL in 100 mL HEX-3-EN-1-YL METHYL CARBONATE, (3Z)- (UNII: WEC5YY1PGT) 0.0004 mL in 100 mL DIMETHYLCYCLOHEXYLETHOXY ISOBUTYLPROPANOATE (UNII: I1AI9W9CAB) 0.0003 mL in 100 mL ETHYL MALTOL (UNII: L6Q8K29L05) 0.0003 mL in 100 mL ALLYL CYCLOHEXANEACETATE (UNII: M6J8835739) 0.00003 mL in 100 mL 1-METHYL-2-(((1R,3S,5S)-1,2,2-TRIMETHYLBICYCLO HEX-3-YL)METHYL)CYCLOPROPANEMETHANOL, (1R,2R)- (UNII: 56FP2ES9B7) 0.00003 mL in 100 mL REISHI (UNII: TKD8LH0X2Z) 0.2 mL in 100 mL HYALURONATE SODIUM (UNII: YSE9PPT4TH) 0.07 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76751-712-02 1 in 1 BLISTER PACK 06/14/2021 1 NDC:76751-712-20 20 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 2 NDC:76751-712-01 90 mL in 1 POUCH; Type 0: Not a Combination Product 06/14/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/14/2021 OLIKA HYDRATING HAND SANITIZER COTTON FLOWER
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76751-711 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65 mL in 100 mL Inactive Ingredients Ingredient Name Strength METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) 0.0548 mL in 100 mL 2,2,5-TRIMETHYLHEXANE (UNII: AGW1Z6985E) 0.0103 mL in 100 mL DIHYDRODEHYDRO-.BETA.-IONONE (UNII: XZS4VD987O) 0.0008 mL in 100 mL 2,4-DIMETHYL-3-CYCLOHEXENE CARBOXALDEHYDE (UNII: 452GFV2AFS) 0.0007 mL in 100 mL .GAMMA.-DECALACTONE (UNII: 7HLS05KP9O) 0.0007 mL in 100 mL .ALPHA.-METHYLBENZYL ACETATE (UNII: FYS3E9NBA3) 0.0007 mL in 100 mL ETHYL 2,2-DIMETHYLHYDROCINNAMAL (UNII: 5V2FN5AA3W) 0.0003 mL in 100 mL 2-ISOBUTYL-4-METHYLTETRAHYDROPYRAN-4-OL (UNII: VK5ZHH2T3F) 0.0685 mL in 100 mL 4-HYDROXY-1-(5-ISOCAMPHYL)CYCLOHEXANE (UNII: TVJ25725XP) 0.0034 mL in 100 mL .GAMMA.-UNDECALACTONE (UNII: QB1T0AG2YL) 0.0007 mL in 100 mL NAPHTHO(2,1-B)FURAN (UNII: 5O098D6W4T) 0.0007 mL in 100 mL 3-HEXENYL ACETATE, (3Z)- (UNII: 6INA6GC5I6) 0.0003 mL in 100 mL DIPROPYLENE GLYCOL (UNII: E107L85C40) 0.1603 mL in 100 mL TETRAMETHYL ACETYLOCTAHYDRONAPHTHALENES (UNII: 2JU6ZH6GRE) 0.0836 mL in 100 mL ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) 0.0753 mL in 100 mL PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) 0.0075 mL in 100 mL IONONE (UNII: QP734LIN1K) 0.0034 mL in 100 mL CYCLOHEXANEPROPANOL, 2,2,6-TRIMETHYL-.ALPHA.-PROPYL- (UNII: CLV4EM4325) 0.0021 mL in 100 mL 2-TERT-BUTYLCYCLOHEXYL ACETATE (UNII: 364FV60913) 0.001 mL in 100 mL ALLYL HEPTANOATE (UNII: AU4CYG9V68) 0.0005 mL in 100 mL REISHI (UNII: TKD8LH0X2Z) 0.2 mL in 100 mL HYALURONATE SODIUM (UNII: YSE9PPT4TH) 0.07 mL in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.25 mL in 100 mL DIHYDROMYRCENOL (UNII: 46L1B02ND9) 0.0199 mL in 100 mL 2-TERT-BUTYLCYCLOHEXYLOXYBUTANOL (UNII: 1DR20642YH) 0.0034 mL in 100 mL JASMONE (UNII: RC4W0G9YUK) 0.0006 mL in 100 mL CYCLOPENTANONE (UNII: 220W81TN3S) 0.0003 mL in 100 mL TRIMETHYLBENZYLSILANE (UNII: HFX8B6Q8WC) 0.0002 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 4.2 mL in 100 mL WATER (UNII: 059QF0KO0R) 29.78 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76751-711-02 1 in 1 BLISTER PACK 06/14/2021 1 NDC:76751-711-20 20 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 2 NDC:76751-711-01 90 mL in 1 POUCH; Type 0: Not a Combination Product 06/14/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/14/2021 Labeler - Olika Inc. (080476192)