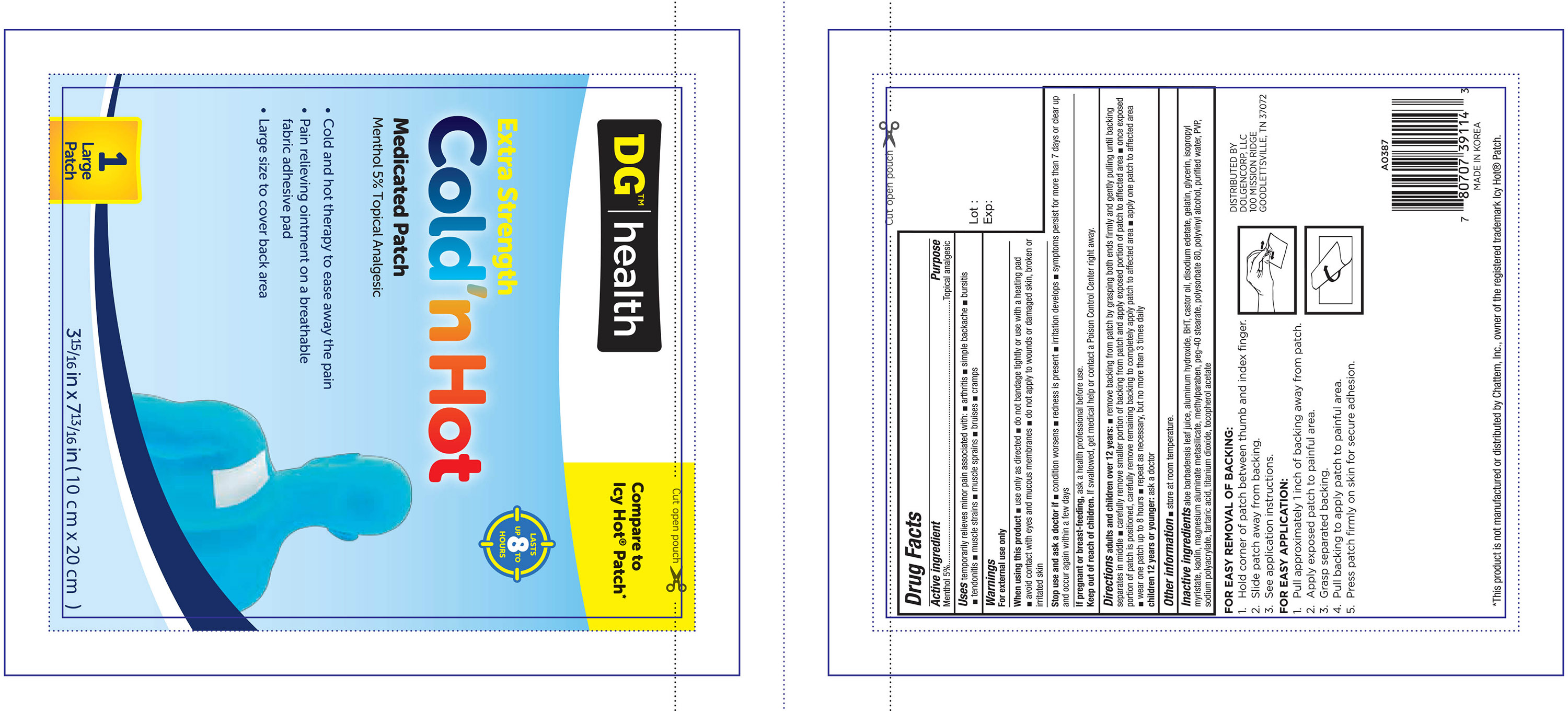
Label: DG HEALTH EXTRA STRENGTH MEDICATED- menthol patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 55910-395-01 - Packager: Dolgencorp Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 1, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
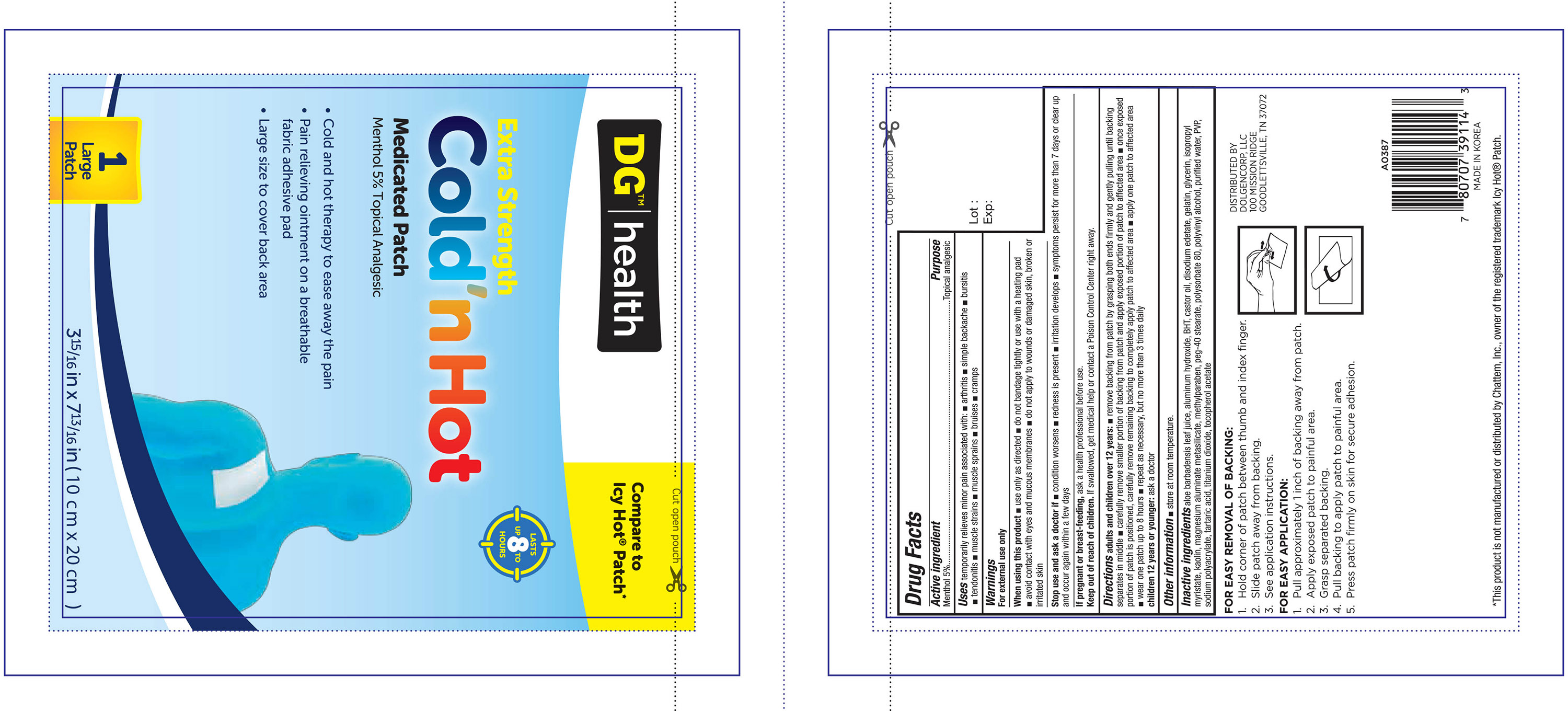
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Directions adults and children over 12 years:
- remove backing from patch by grasping both ends firmly and gently pulling until backing separates in middle
- carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
- apply one patch to affected area
- wear one patch up to 8 hours
- repeat as necessary, but no more than 3 times daily
Children 12 years or younger: ask a doctor
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
aloe barbadensis leaf juice, aluminum hydroxide, BHT, castor oil, disodium edetate, gelatin, glycerin, isopropyl myristate, kaolin, magnesium
aluminate metasilicate, methylparaben, peg-40 stearate, polysorbate 80, polyvinyl alcohol, purified water, PVP, sodium polyacrylate, tartaric
acid, titanium dioxide, tocopherol acetate
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DG HEALTH EXTRA STRENGTH MEDICATED
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55910-395 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 750 mg Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CASTOR OIL (UNII: D5340Y2I9G) EDETATE DISODIUM (UNII: 7FLD91C86K) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) KAOLIN (UNII: 24H4NWX5CO) SILODRATE (UNII: 9T3UU8T0QK) METHYLPARABEN (UNII: A2I8C7HI9T) PEG-40 STEARATE (UNII: ECU18C66Q7) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) WATER (UNII: 059QF0KO0R) POVIDONES (UNII: FZ989GH94E) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55910-395-01 1 in 1 CARTON 06/30/2013 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/30/2013 Labeler - Dolgencorp Inc (068331990)