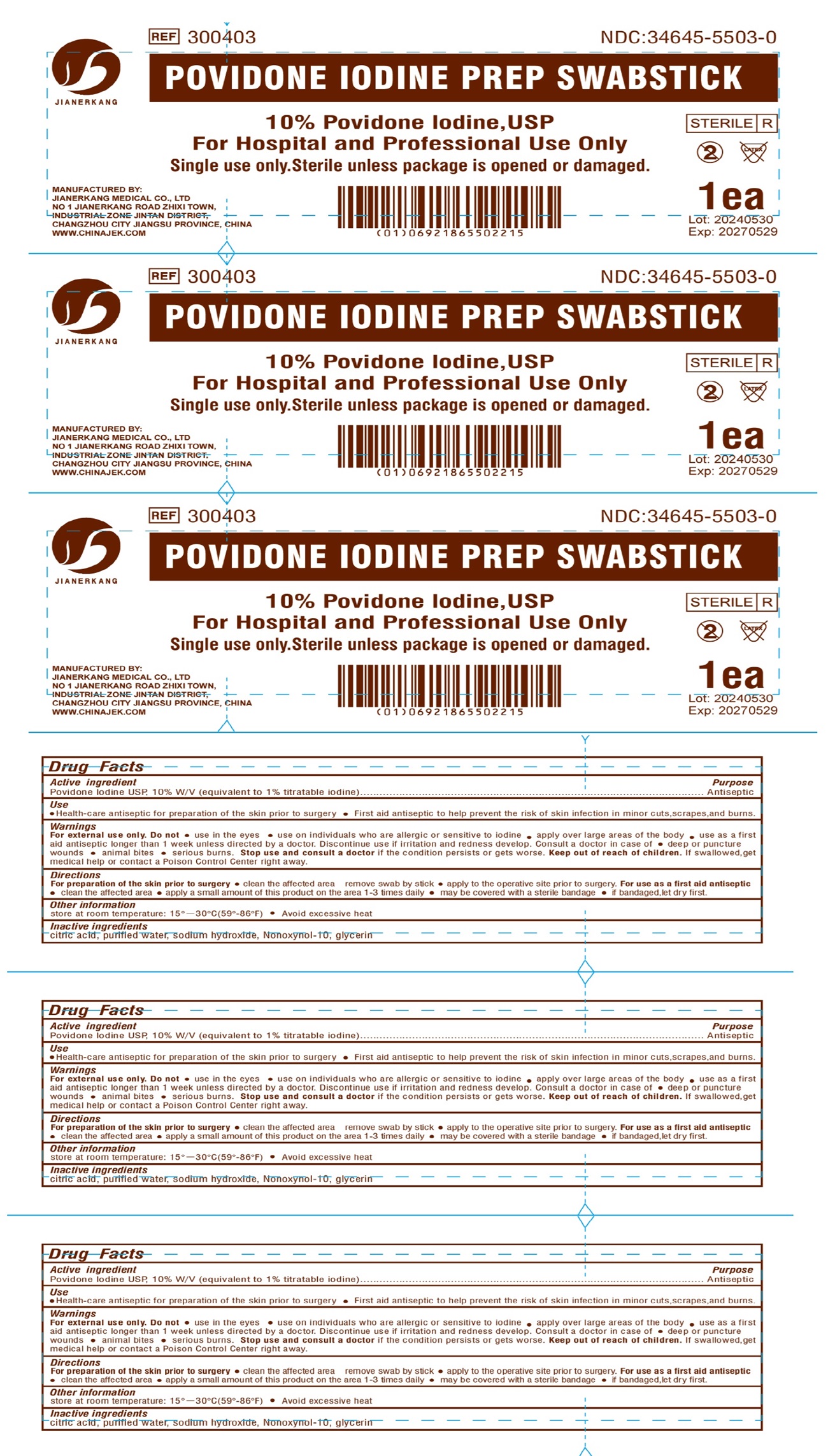
Label: POVIDONE IODINE PREP- povidone-iodine swab
- NDC Code(s): 34645-5506-0, 34645-5506-1
- Packager: Jianerkang Medical Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use
-
Warnings
For external use only.
Do not
- use in the eyes
- use on individuals who are allergic or sensitive to iodine
- apply over large areas of the body
- use as a first aid antiseptic longer than 1 week unless directed by a doctor. Discontinue use if irritation and irritation and redness develop. Consult a doctor in case of
- deep or puncture wounds
- animal bites
- serious burns.
-
Directions
For preparation of the skin prior to surgery
- clean the affected area remove swab by stick
- apply to the operative site prior to surgery.
For use as a first aid antiseptic
- clean the affected area
- apply a small amount of this product on the area 1-3 times daily
- may be covered with a sterile bandage
- if bandaged,let dry first.
- Other information
- Inactive ingredients
- 34645-5506-0
- 34645-5506-1
-
INGREDIENTS AND APPEARANCE
POVIDONE IODINE PREP
povidone-iodine swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:34645-5506 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 100 mg in 1 g Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) NONOXYNOL-10 (UNII: K7O76887AP) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:34645-5506-0 500 in 1 CARTON 08/01/2019 1 1 in 1 POUCH 1 2.5 g in 1 POUCH; Type 0: Not a Combination Product 2 NDC:34645-5506-1 250 in 1 CARTON 08/01/2019 2 3 in 1 POUCH 2 13.5 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2019 Labeler - Jianerkang Medical Co., Ltd (530968767) Establishment Name Address ID/FEI Business Operations Jianerkang Medical Co., Ltd 530968767 manufacture(34645-5506) , label(34645-5506)