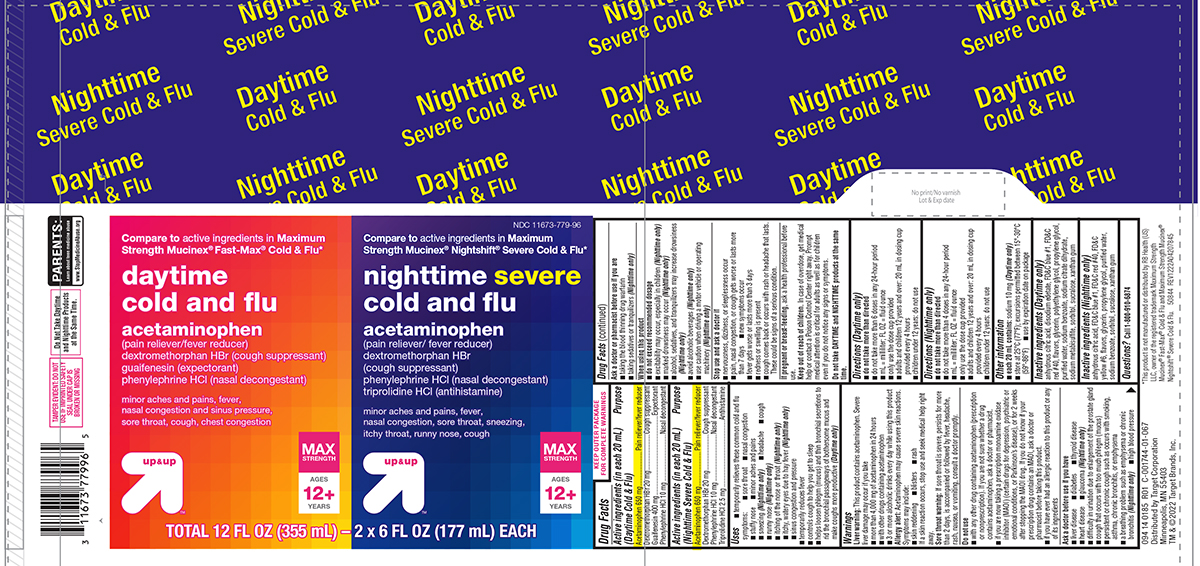
Label: COLD AND FLU DAYTIME NIGHTTIME- acetaminophen, dextromethorphan hbr, guaifenesin, phenylephrine hcl, triprolidine hcl kit
- NDC Code(s): 11673-779-96, 11673-885-45, 11673-889-45
- Packager: Target Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 20 mL) (Daytime Cold & Flu)
- Purpose
- Active ingredients (in each 20 mL) (Nighttime Severe Cold & Flu)
- Purpose
-
Uses
- temporarily relieves these common cold and flu symptoms:
- sore throat
- nasal congestion
- stuffy nose
- minor aches and pains
- sneezing (Nighttime only)
- headache
- cough
- runny nose (Nighttime only)
- itching of the nose or throat (Nighttime only)
- itchy, watery eyes due to hay fever (Nighttime only)
- sinus congestion and pressure
- sore throat
- temporarily reduces fever
- controls cough to help you get to sleep
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive (Daytime only)
- temporarily relieves these common cold and flu symptoms:
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- diabetes
- thyroid disease
- heart disease
- glaucoma (Nighttime only)
- difficulty in urination due to enlargement of the prostate gland
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- a breathing problem such as emphysema or chronic bronchitis (Nighttime only)
- high blood pressure
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers (Nighttime only)
When using this product
-
do not exceed recommended dosage
- excitability may occur, especially in children (Nighttime only)
- marked drowsiness may occur (Nighttime only)
- alcohol, sedatives, and tranquilizers may increase drowsiness (Nighttime only)
- avoid alcoholic beverages (Nighttime only)
- use caution when driving a motor vehicle or operating machinery (Nighttime only)
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain, nasal congestion, or cough gets worse or lasts more than 7 days
- new symptoms occur
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
- Directions (Daytime only)
- Directions (Nighttime only)
- Other information
- Inactive ingredients (Daytime only)
- Inactive ingredients (Nighttime only)
- Questions?
-
Principal display panel
Compare to active ingredients in Maximum
Strength Mucinex®Fast-Max®Cold & Flu*
daytime
cold and flu
acetaminophen
(pain reliever/fever reducer)
dextromethorphan HBr (cough suppressant)
guaifenesin (expectorant)
phenylephrine HCl (nasal decongestant)
minor aches and pains, fever,
nasal congestion and sinus pressure,
sore throat, cough, chest congestion
up & up™MAX STRENGTH
AGES
12+
YEARSNDC 11673-779-96
Compare to active ingredients in Maximum
Strength Mucinex® Nightshift® Severe Cold & Flu*nighttime severe
cold and fluacetaminophen
(pain reliever/fever reducer)
dextromethorphan HBr
(cough suppressant)
phenylephrine HCl (nasal decongestant)
triprolidine HCl (antihistamine)minor aches and pains, fever,
nasal congestion, sore throat, sneezing,
itchy throat, runny nose, cough
up & up™MAX
STRENGTHAGES
12+
YEARSTOTAL 12 FL OZ (355 mL) - 2 x 6 FL OZ (177 mL) EACH
TAMPER EVIDENT: DO NOT
USE IF IMPRINTED SAFETY
SEAL UNDER CAP IS
BROKEN OR MISSINGPARENTS:
Learn about teen medicine abuse
www.StopMedicineAbuse.orgDo Not Take Daytime
and Nighttime Products
at the Same Time.094 14 0185 R01 C-001744-01-067
Distributed by Target Corporation
Minneapolis, MN 55403
TM & ©2022 Target Brands, Inc.*This product is not manufactured or distributed by RB Health (US)
LLC, owner of the registered trademark Maximum Strength
Mucinex® Fast-Max® Cold & Flu and Maximum Strength Mucinex®
Nightshift® Severe Cold & Flu. 50844 REV1222A04207845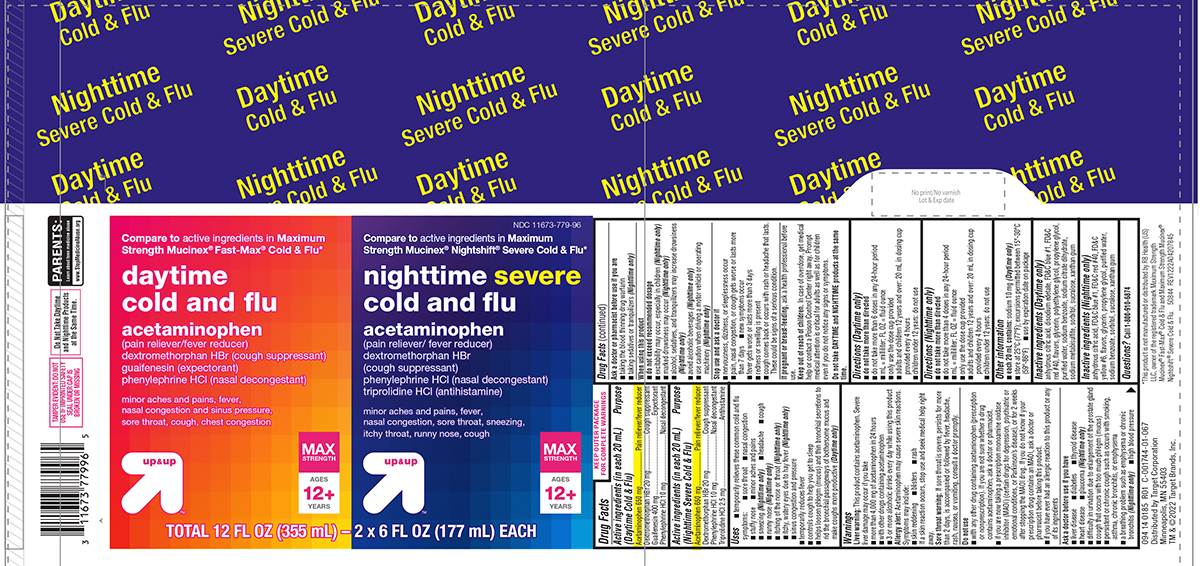
Target 44-042078
-
INGREDIENTS AND APPEARANCE
COLD AND FLU DAYTIME NIGHTTIME
acetaminophen, dextromethorphan hbr, guaifenesin, phenylephrine hcl, triprolidine hcl kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-779 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-779-96 1 in 1 PACKAGE; Type 0: Not a Combination Product 05/17/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 177 mL Part 2 1 BOTTLE, PLASTIC 177 mL Part 1 of 2 COLD AND FLU DAYTIME
acetaminophen, dextromethorphan hbr, guaifenesin, phenylephrine hcl solutionProduct Information Item Code (Source) NDC:11673-885 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 20 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg in 20 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color blue Score Shape Size Flavor BERRY (MIXED) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-885-45 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/17/2022 Part 2 of 2 COLD AND FLU NIGHTTIME
acetaminophen, dextromethorphan hbr, phenylephrine hcl, triprolidine hcl solutionProduct Information Item Code (Source) NDC:11673-889 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 20 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg in 20 mL TRIPROLIDINE HYDROCHLORIDE (UNII: YAN7R5L890) (TRIPROLIDINE - UNII:2L8T9S52QM) TRIPROLIDINE HYDROCHLORIDE 2.5 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color blue Score Shape Size Flavor FRUIT (MIXED) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-889-45 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/17/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/17/2022 Labeler - Target Corporation (006961700) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 manufacture(11673-779) , pack(11673-779)