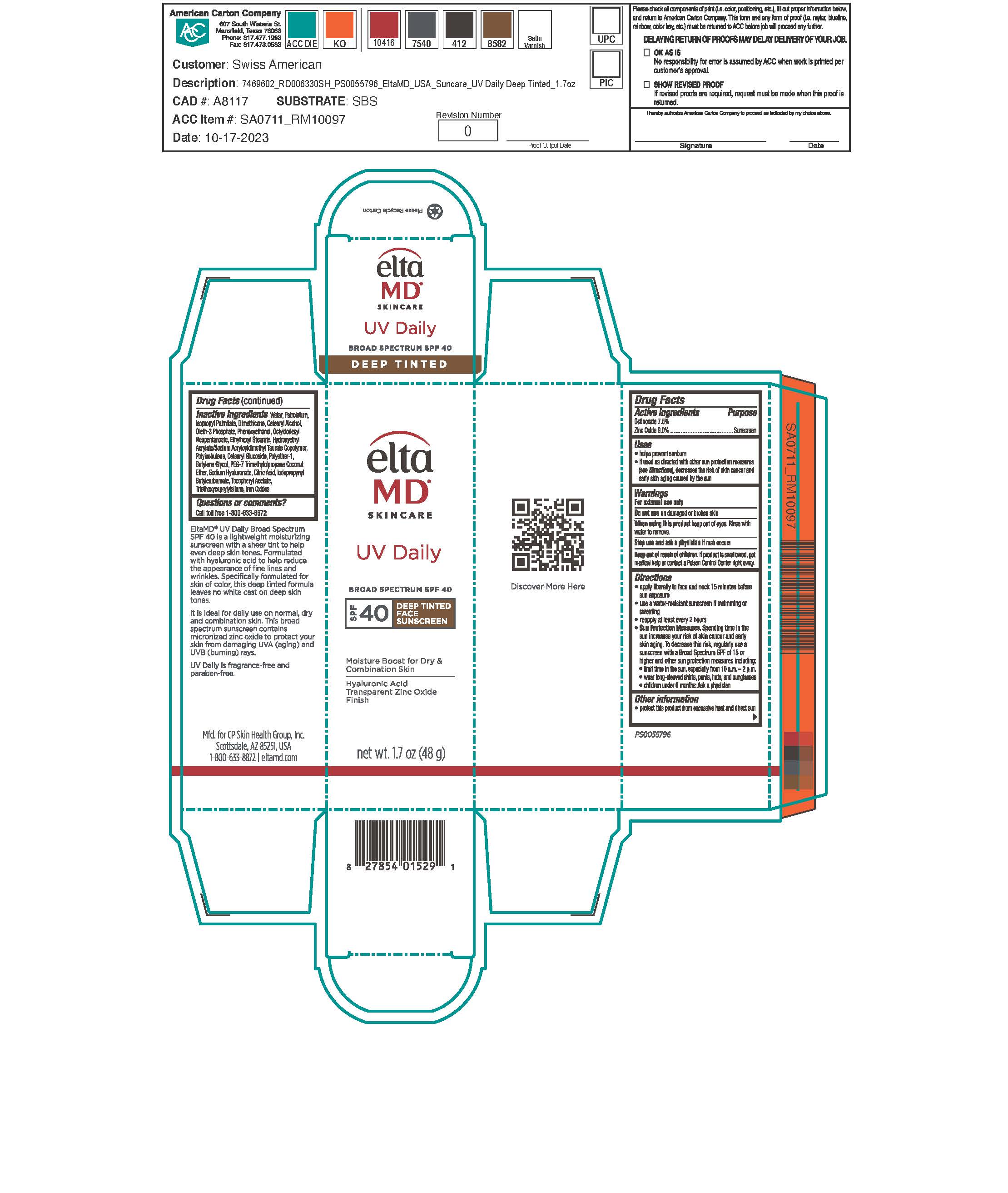
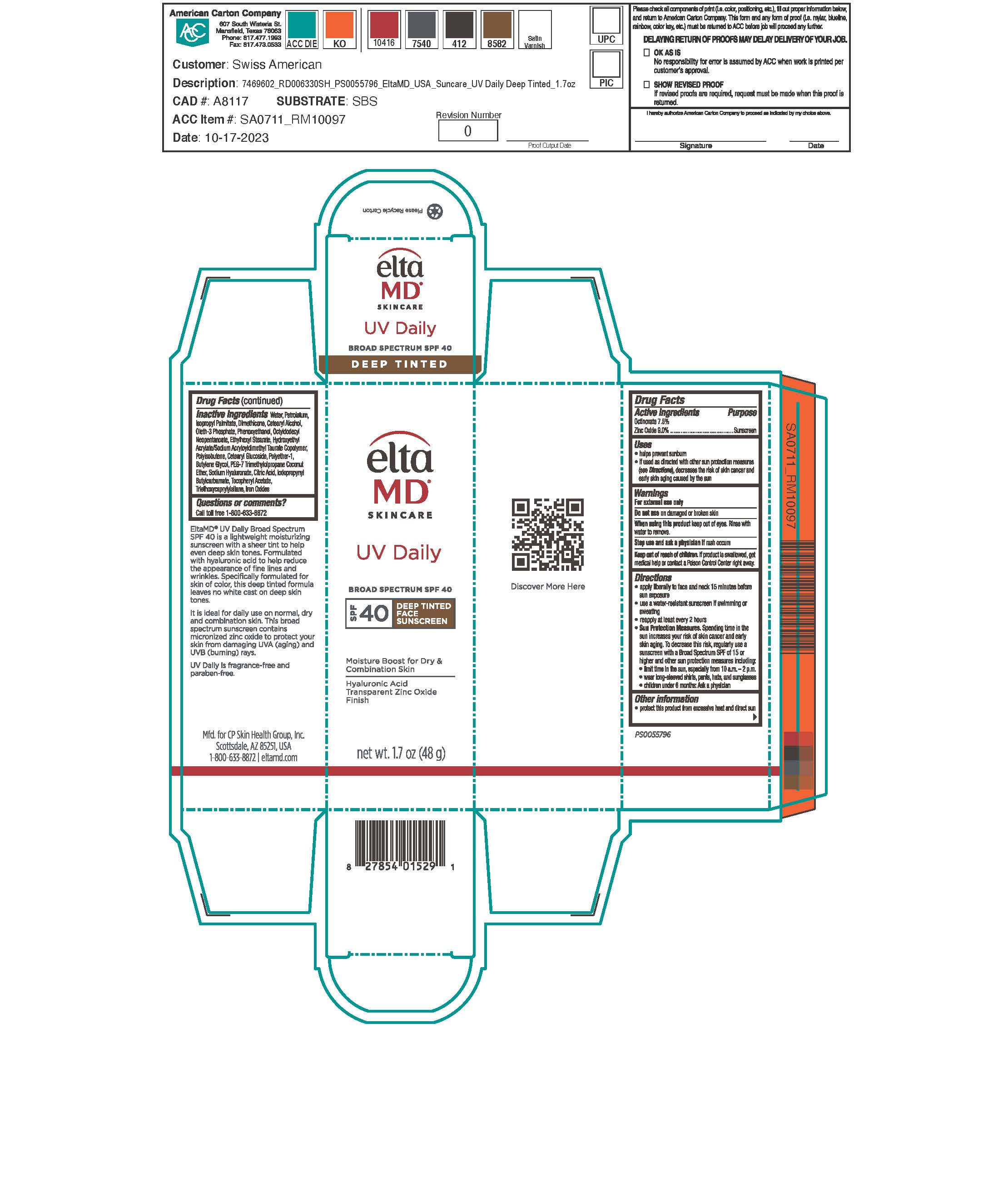
Label: UV DAILY DEEP TINT- octinoxate, zinc oxide sunscreen lotion
- NDC Code(s): 72043-2920-1, 72043-2920-2
- Packager: CP Skin Health Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warnings
- Keep out of reach of children
- Uses
- Uses
-
Directions
Apply liberally 15 minutes before sun exposure. Use a water-resistant sunscreen if swimming or sweating. Reapply at least every 2 hours. Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 am to 2 pm. Wear long-sleeve shirts, pants, hats, and sunglasses. Children under 6 months: ask a physician.
- Active Ingredients
-
Inactive Ingredients
water, petrolatum, isopropyl palmitate, dimethicone, cetearyl alcohol, oleth-3 phosphate, phenoxyethanol, octyldodecyl neopentanoate, ethylhexyl stearate, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, polyisobutene, cetearyl glucoside, polyether-1, butylene glycol, PEG-7 trimethylolpropane cocnut ether, sodium hyaluronate, citric acid, iodopropynyl butylcarbamate, tocopheryl acetate, triethoxycaprylylsilane, iorn oxides
- Questions
- Labeling
-
INGREDIENTS AND APPEARANCE
UV DAILY DEEP TINT
octinoxate, zinc oxide sunscreen lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72043-2920 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 750 g in 1000 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 900 g in 1000 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE (UNII: 92RU3N3Y1O) PETROLATUM (UNII: 4T6H12BN9U) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) OLETH-3 PHOSPHATE (UNII: 8Q0Z18J1VL) PHOSPHORIC ACID (UNII: E4GA8884NN) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONIC ACID (UNII: S270N0TRQY) PEG-7 TRIMETHYLOLPROPANE COCONUT ETHER (UNII: MVJ3AD73GG) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) Product Characteristics Color brown Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72043-2920-1 50 g in 1 BOTTLE; Type 0: Not a Combination Product 05/09/2024 2 NDC:72043-2920-2 2 g in 1 PACKET; Type 0: Not a Combination Product 05/09/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/09/2024 Labeler - CP Skin Health Group, Inc. (611921669) Registrant - Swiss-American CDMO, LLC (080170933) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(72043-2920)