Label: SPF BEST SELLERS MINI KIT- avobenzone, homosalate, octisalate, octocrylene kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 75936-258-01, 75936-259-01, 75936-260-01, 75936-261-01, view more75936-262-01 - Packager: TAYLOR JAMES, LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 16, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
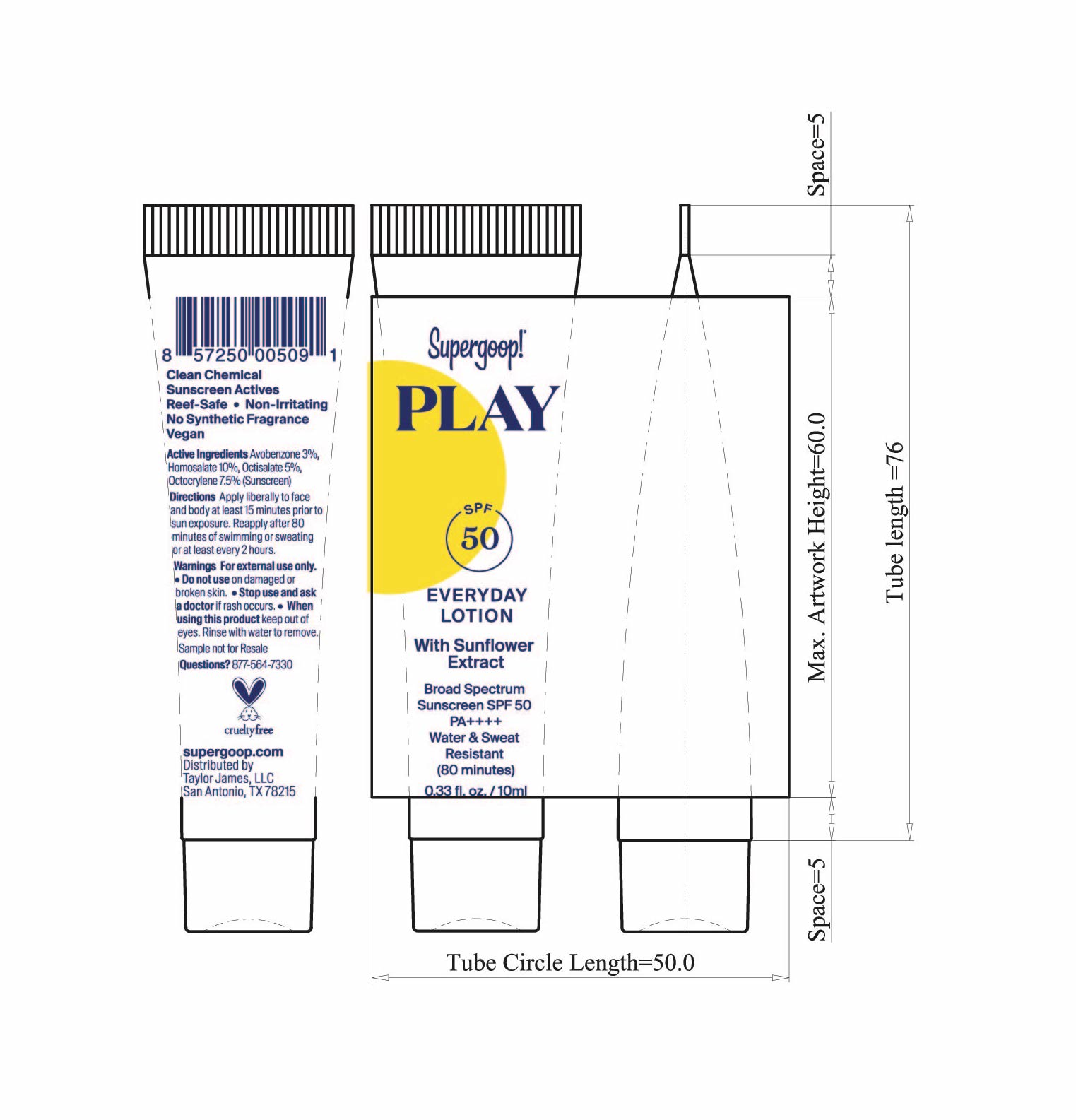
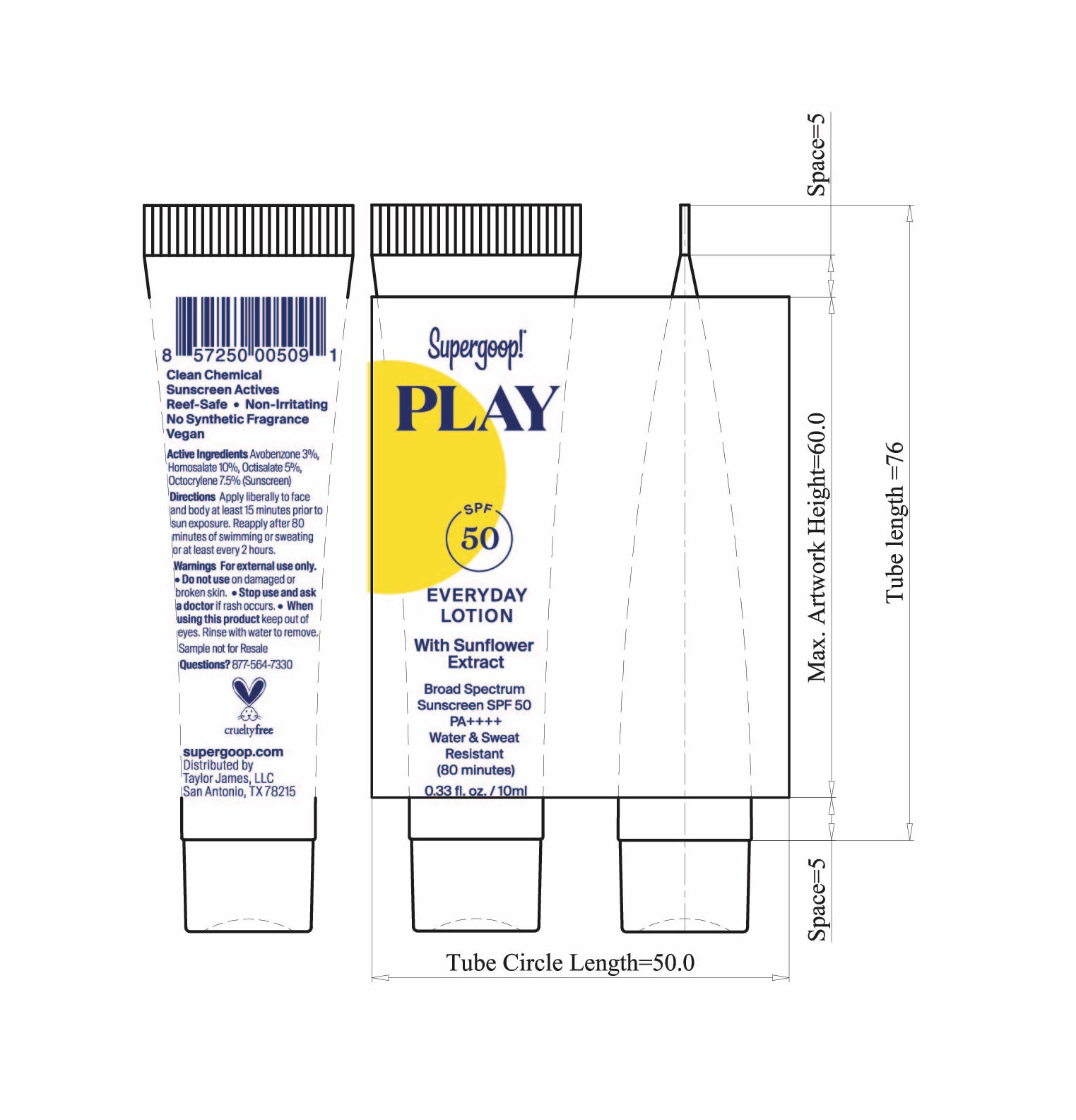
- Play Everyday Sunscreen SPF 50 with Sunflower extract Active ingredients
- Unseen Sunscreen SPF 40 active ingredients
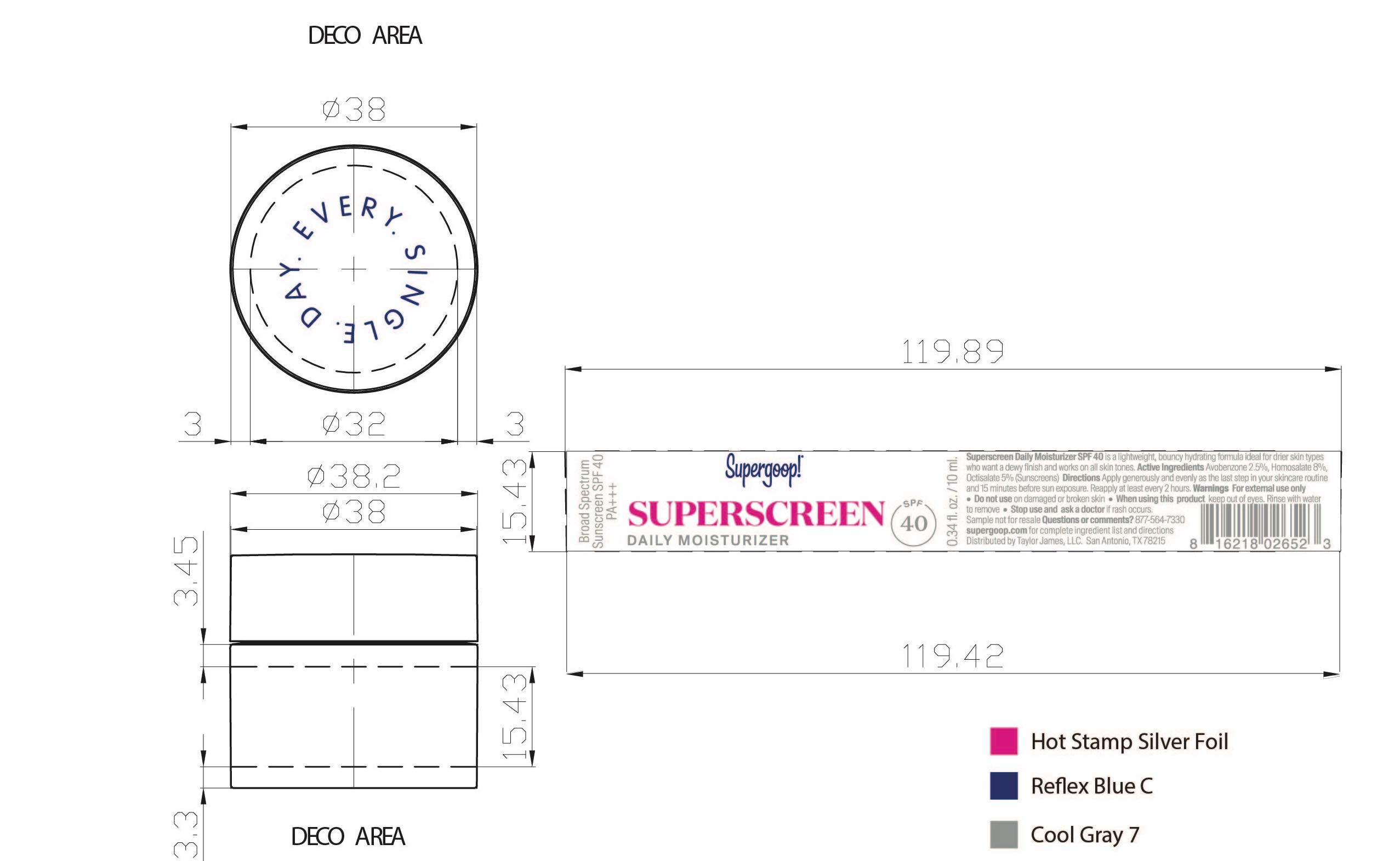
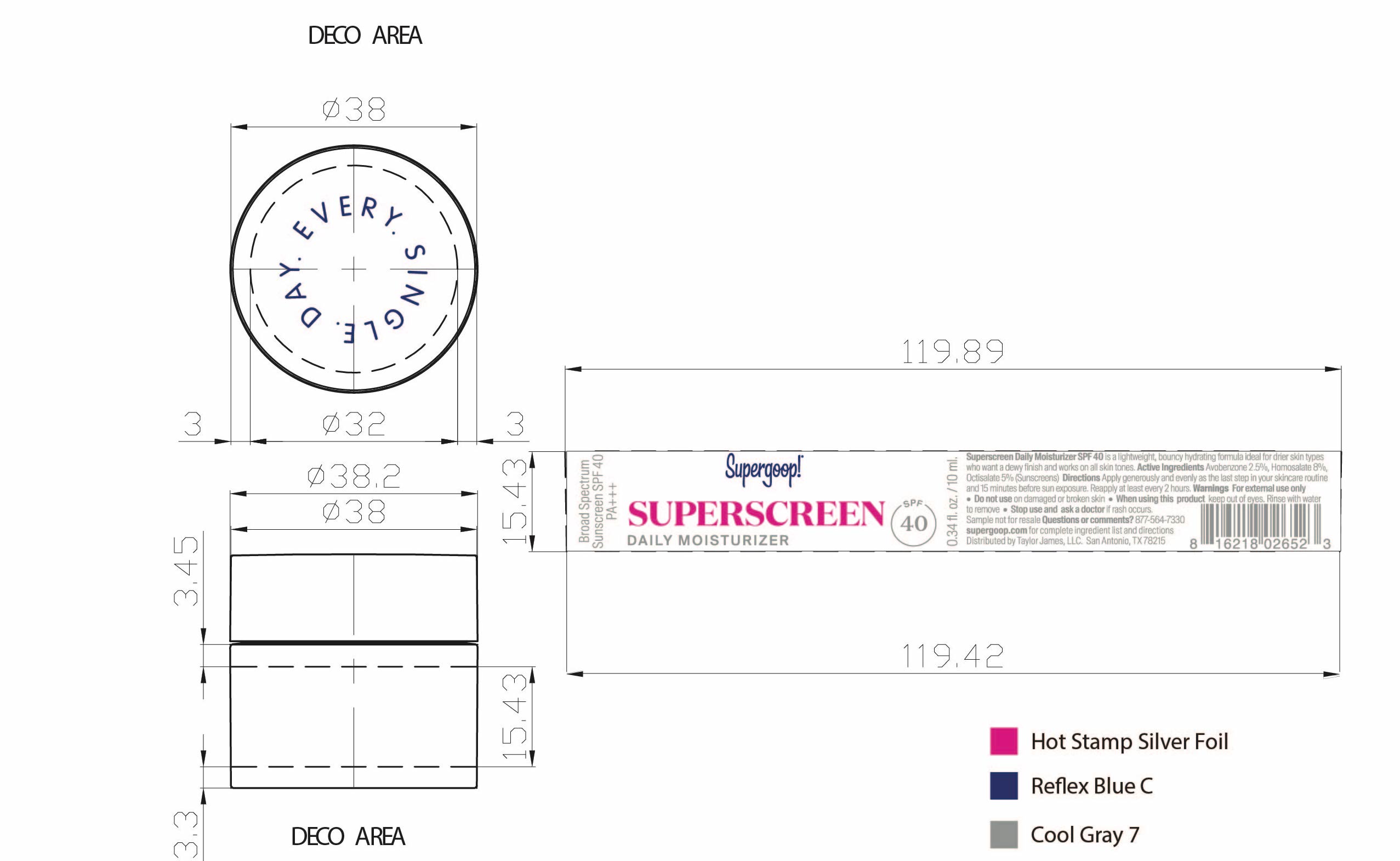
- Superscreen Daily Moisturizer SPF 40
- Glow Screen SPF 40
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
Play Everyday Lotion SPF 50 with Sunflower Extract Directions
Directions
- Apply generously and evenly 15 minutes before sun exposure
- Reapply:after 80 minutes of swimming or sweating
immediately after towel drying
at least every 2 hours- Sun Protection Measures. Spending time in the sun increases your risk of skin
cancer and early skin aging. To decrease this risk, regularly use a sunscreen
with broad spectrum SPF of 15 or higher and other sun protection measures
including:- limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear Long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
-
DOSAGE & ADMINISTRATION
Directions
- Apply generously and evenly 15 minutes before sun exposure
- Reapply:after 40 minutes of swimming or sweating
immediately after towel drying
at least every 2 hours- Sun Protection Measures. Spending time in the sun increases your risk of skin
cancer and early skin aging. To decrease this risk, regularly use a sunscreen
with broad spectrum SPF of 15 or higher and other sun protection measures
including:- limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear Long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
-
Unseen Sunscreen SPF 40 Inactive Ingredients
Inactive Ingredients
Isododecane, Dimethicone Crosspolymer, Dimethicone/Bis-Isobutyl PPG-20 Crosspolymer, Polymethylsilsesquioxane, Isohexadecane, Dicrapylyl Carbonate, Meadowfoam Estolide, Caprylic/Capric Triglyceride, Polyester-7, Neopentyl Glycol Diheptanoate, Lithothamnion Calcareum Extract,Butyrospermum Parkii (Shea) Butter, Jojoba Esters, Mannitol, Boswellia Serrata Resin Extract, Lecithin, Microcrystalline Cellulose, Diatomaceous Earth, Zinc Sulfate, Silica, Tocopherol
-
Sunscreen Daily Moisturizer SPF 40 Inactive Ingredients
Inactive ingredients Water, Cartamus Tinctorius (safflower) Olesomes, Glycerin, C12-15 Alkyl Benzoate, Polyester-7, Ammonium Acryloyldimethyldimethyltaurate/VP Copolymer, Neopentyl Glycol Diheptanoate, Carthamus Tinctorius (Safflower) Seedcake extract, Aphanizomenon Flos-Aquae Extract, Inulin Lauryl Carbamate, Xanthan Gum, Biosaccharide Gum-4, Cerium Oxide, Platinum Powder, 1,2-hexanediol, Ethylhexylglycerin, Phenethyl Alcohol, Chlorphenesin
-
Play Everyday Sunscreen SPF 50 with sunflower Inactive ingredients
Inactive ingredients
Water, Acrylates Copolymer, Diisopropyl Sebacate, Glycerin, Isodecyl Neopentanoate, Isododecane, Lauryl Lactate, Cetyl Alcohol, Potassium Cetyl Phosphate, Brassica Camprestris/Aleurites Fordi Copolymer, Oryza Sativa (Rice) Bran Extract, Cetearyl Olivate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Hydroxyacetophenone, Sorbitan Olivate, Diethylhexyl Syringylidenemalonate, Aniba Rosaeodora (Rosewood) Wood Oil, Chlorphenesin, Citrus Aurantium Dulcis (Orange) Peel Oil, Citrus Limon (Lemon) Peel Oil, Ethylhexylglycerin, Eucalyptus Globulus Leaf Oil, Ocimum Bascilicum (Basil) Flower/Leaf Extract, Pelargonium Graveolens Flower Oil, Pogostemon Cablin Oil, Pentylene Glycol, 1,2-hexanediol, Caprylyl Glycol, Xanthan Gum, Helianthus Annuus (Sunflower) Extract, Behenic Acid, Cetyl Behenate, Isostearyl Isostearate, Trisodium Ethylenediamene Disuccinate, Tocopherol, Allantoin, Rosmarinus Officinalis (Rosemary) Leaf Extract, Caprylic/Capric Triglyceride,Panthenol, Pentasodium Triphosphate, Citric Acid
-
Glowscreen SPF 40 Inactive ingredients
Inactive Ingredients
Water, Propanediol, Butyloctyl Salicylate, Glycerin, C12-15 Alkyl Benzoate, Polymethylsilsesquioxane, Niacinamide, Glyceryl Stearate Citrate, Bismuth Oxychloride, Mica, Titanium Dioxide, Lauryl Lactate, Isododecane, Isodecyl Neopentanoate, Glyceryl Stearate Citrate, Bismuth Oxychloride, Mica, Titanium Dioxide, Lauryl Lactate, Isododecane, Isodecyl Neopentanoate, Glyceryl Stearate, Diisopropyl Sebacate, Cetyl Phosphate, Caprylic/Capric Triglyceride, Coco-Caprylate, Ethylhexyl Hydroxystearate, Butylene Glycol, Arginine, Hydroxyacetophenone, Caprylyl Glycol, 1,2-Hexanediol, Iron Oxides, Sodium Hyaluronate, Chlorphenesin, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Trisodium Ethylenediamine Disuccinate, Phospholipids, Limonium Gerberi Extract, Leuconostoc/Radish Root Ferment Filtrate, Theobroma Cocoa (Cocoa) Seed Extract, Pantothenic Acid , Tocopherol, Helianthus Annuus (Sunflower) Seed Oil, Ferulic Acid
- Bestseller Kit
- Unseen Sunscreen tube
- Superscreen Daily Moisturizer SPF 40
- PLAY Everyday Lotion SPF 50 Sunflower extract tube
- Glowscreen SPF 40 tube
-
INGREDIENTS AND APPEARANCE
SPF BEST SELLERS MINI KIT
avobenzone, homosalate, octisalate, octocrylene kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-258 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-258-01 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 04/19/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 10 mL Part 2 1 BOTTLE, SPRAY 10 mL Part 3 1 TUBE 10 mL Part 4 1 TUBE 10 mL Part 1 of 4 UNSEEN SUNSCREEN SPF 40
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Item Code (Source) NDC:75936-260 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 8 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHYMATOLITHON CALCAREUM (UNII: 6J1M3WA0ZK) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SHEA BUTTER (UNII: K49155WL9Y) MANNITOL (UNII: 3OWL53L36A) TOCOPHEROL (UNII: R0ZB2556P8) ISODODECANE (UNII: A8289P68Y2) DIATOMACEOUS EARTH (UNII: 2RF6EJ0M85) ZINC SULFATE (UNII: 89DS0H96TB) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) POLYESTER-7 (UNII: 0841698D2F) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) ISOHEXADECANE (UNII: 918X1OUF1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-260-01 10 mL in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/22/2021 Part 2 of 4 GLOWSCREEN SPF 40
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Item Code (Source) NDC:75936-261 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) PANTOTHENIC ACID (UNII: 19F5HK2737) SUNFLOWER OIL (UNII: 3W1JG795YI) GLYCERIN (UNII: PDC6A3C0OX) TOCOPHEROL (UNII: R0ZB2556P8) FERULIC ACID (UNII: AVM951ZWST) CHLORPHENESIN (UNII: I670DAL4SZ) CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) COCOA (UNII: D9108TZ9KG) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ETHYLHEXYL HYDROXYSTEARATE (UNII: B7I80BVV5E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) PROPANEDIOL (UNII: 5965N8W85T) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ARGININE (UNII: 94ZLA3W45F) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) WATER (UNII: 059QF0KO0R) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) LAURYL LACTATE (UNII: G5SU0BFK7O) ISODODECANE (UNII: A8289P68Y2) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) CETYL PHOSPHATE (UNII: VT07D6X67O) COCO-CAPRYLATE (UNII: 4828G836N6) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-261-01 10 mL in 1 BOTTLE, SPRAY; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/19/2021 Part 3 of 4 PLAY EVERYDAY SPF 50 WITH SUNFLOWER EXTRACT
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Item Code (Source) NDC:75936-262 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7.5 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) POGOSTEMON CABLIN LEAF OIL (UNII: F3IN55X5PO) PENTYLENE GLYCOL (UNII: 50C1307PZG) ALLANTOIN (UNII: 344S277G0Z) BEHENIC ACID (UNII: H390488X0A) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) EUCALYPTUS OIL (UNII: 2R04ONI662) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) CETYL BEHENATE (UNII: WFM51TRO3E) RICE BRAN (UNII: R60QEP13IC) CETEARYL OLIVATE (UNII: 58B69Q84JO) ROSEWOOD OIL (UNII: F2522O5L7B) CHLORPHENESIN (UNII: I670DAL4SZ) ROSEMARY (UNII: IJ67X351P9) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SODIUM TRIPOLYPHOSPHATE ANHYDROUS (UNII: 9SW4PFD2FZ) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) XANTHAN GUM (UNII: TTV12P4NEE) ISOSTEARYL ISOSTEARATE (UNII: IV0Z586Z4Y) TOCOPHEROL (UNII: R0ZB2556P8) CETYL ALCOHOL (UNII: 936JST6JCN) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) ISODODECANE (UNII: A8289P68Y2) SORBITAN OLIVATE (UNII: MDL271E3GR) PANTHENOL (UNII: WV9CM0O67Z) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) LAURYL LACTATE (UNII: G5SU0BFK7O) ORANGE OIL, COLD PRESSED (UNII: AKN3KSD11B) OCIMUM BASILICUM FLOWERING TOP (UNII: 7SAB275FP2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-262-01 10 mL in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/19/2021 Part 4 of 4 SUPERSCREEN DAILY MOISTURIZER SPF 40
avobenzone, homosalate, octisalate creamProduct Information Item Code (Source) NDC:75936-259 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 8 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.5 g in 100 mL Inactive Ingredients Ingredient Name Strength CARTHAMUS TINCTORIUS SEED OLEOSOMES (UNII: 9S60Q72309) XANTHAN GUM (UNII: TTV12P4NEE) PLATINUM (UNII: 49DFR088MY) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CERIC OXIDE (UNII: 619G5K328Y) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) POLYESTER-7 (UNII: 0841698D2F) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) CARTHAMUS TINCTORIUS SEEDCAKE (UNII: DHQ13F4N2W) APHANIZOMENON FLOSAQUAE (UNII: 49VG1X560X) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-259-01 10 mL in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 05/10/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/19/2021 Labeler - TAYLOR JAMES, LTD. (033381850)