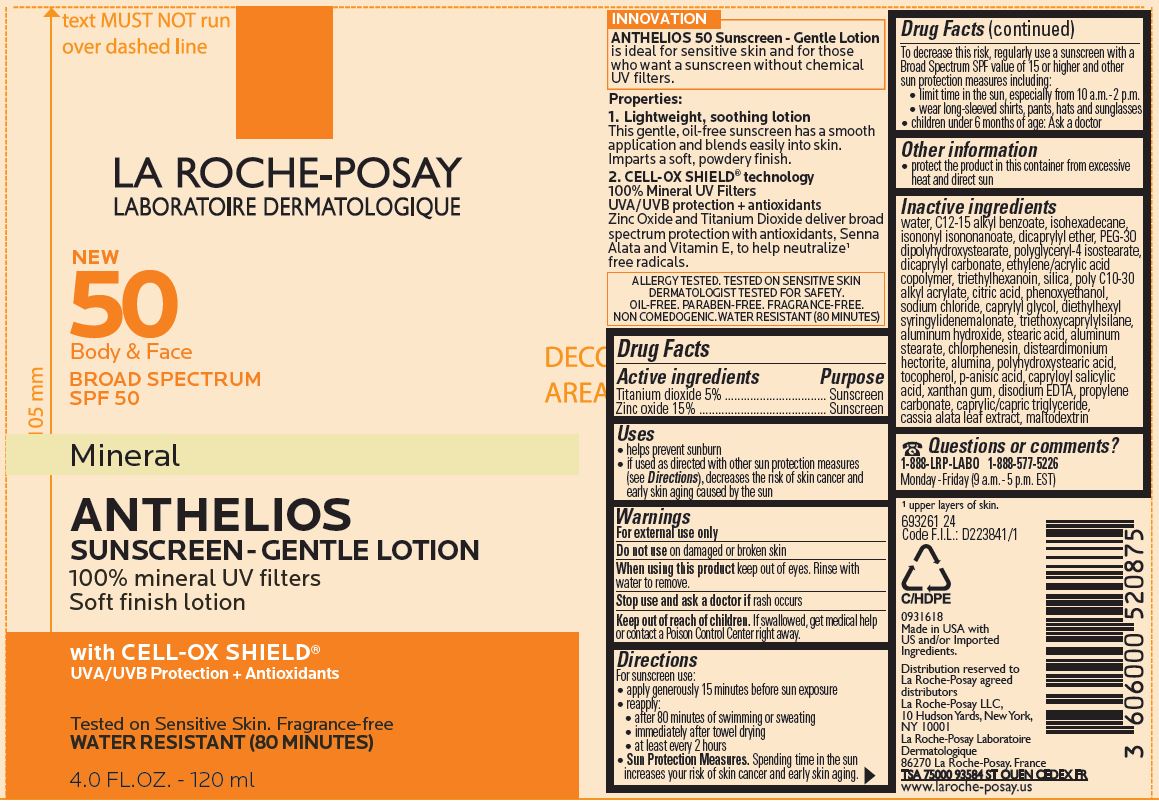
Label: LA ROCHE POSAY NEW 50 BODY AND FACE SPF 50 MINERAL ANTHELIOS GENTLE 100 PERCENT MINERAL UV FILTERS SOFT FINISH WITH CELL OX SHIELD- titanium dioxide and zinc oxide lotion
-
NDC Code(s):
49967-091-01,
49967-091-02,
49967-091-03,
49967-091-04, view more49967-091-05
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
For sunscreen use:
- apply generously 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, C12-15 alkyl benzoate, isohexadecane, isononyl isononanoate, dicaprylyl ether, PEG-30 dipolyhydroxystearate, polyglyceryl-4 isostearate, dicaprylyl carbonate, ethylene/acrylic acid copolymer, triethylhexanoin, silica, poly C10-30 alkyl acrylate, citric acid, phenoxyethanol, sodium chloride, caprylyl glycol, diethylhexyl syringylidenemalonate, triethoxycaprylylsilane, aluminum hydroxide, stearic acid, aluminum stearate, chlorphnesin, disteardimonium hectorite, alumina, polyhydroxystearic acid, tocopherol, p-ansic acid, capryloyl salicylic acid, xanthan gum, disodium EDTA, propylene carbonate, caprylic/capric triglyceride, cassia alata leaf extract, maltodextrin
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LA ROCHE POSAY NEW 50 BODY AND FACE SPF 50 MINERAL ANTHELIOS GENTLE 100 PERCENT MINERAL UV FILTERS SOFT FINISH WITH CELL OX SHIELD
titanium dioxide and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-091 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 50 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ISOHEXADECANE (UNII: 918X1OUF1E) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) DICAPRYLYL ETHER (UNII: 77JZM5516Z) PEG-30 DIPOLYHYDROXYSTEARATE (UNII: 9713Q0S7FO) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CHLORIDE (UNII: 451W47IQ8X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM STEARATE (UNII: U6XF9NP8HM) CHLORPHENESIN (UNII: I670DAL4SZ) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM OXIDE (UNII: LMI26O6933) TOCOPHEROL (UNII: R0ZB2556P8) P-ANISIC ACID (UNII: 4SB6Y7DMM3) CAPRYLOYL SALICYLIC ACID (UNII: 5F7PJF6AA4) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLENE CARBONATE (UNII: 8D08K3S51E) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SENNA ALATA LEAF (UNII: 4BXR6YZN92) MALTODEXTRIN (UNII: 7CVR7L4A2D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-091-01 120 mL in 1 TUBE; Type 0: Not a Combination Product 12/15/2018 2 NDC:49967-091-02 90 mL in 1 TUBE; Type 0: Not a Combination Product 12/15/2018 3 NDC:49967-091-03 5 mL in 1 PACKET; Type 0: Not a Combination Product 12/15/2018 4 NDC:49967-091-04 5 mL in 1 TUBE; Type 0: Not a Combination Product 12/15/2018 5 NDC:49967-091-05 150 mL in 1 TUBE; Type 0: Not a Combination Product 12/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/15/2018 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'OREAL USA, INC. 185931458 manufacture(49967-091)