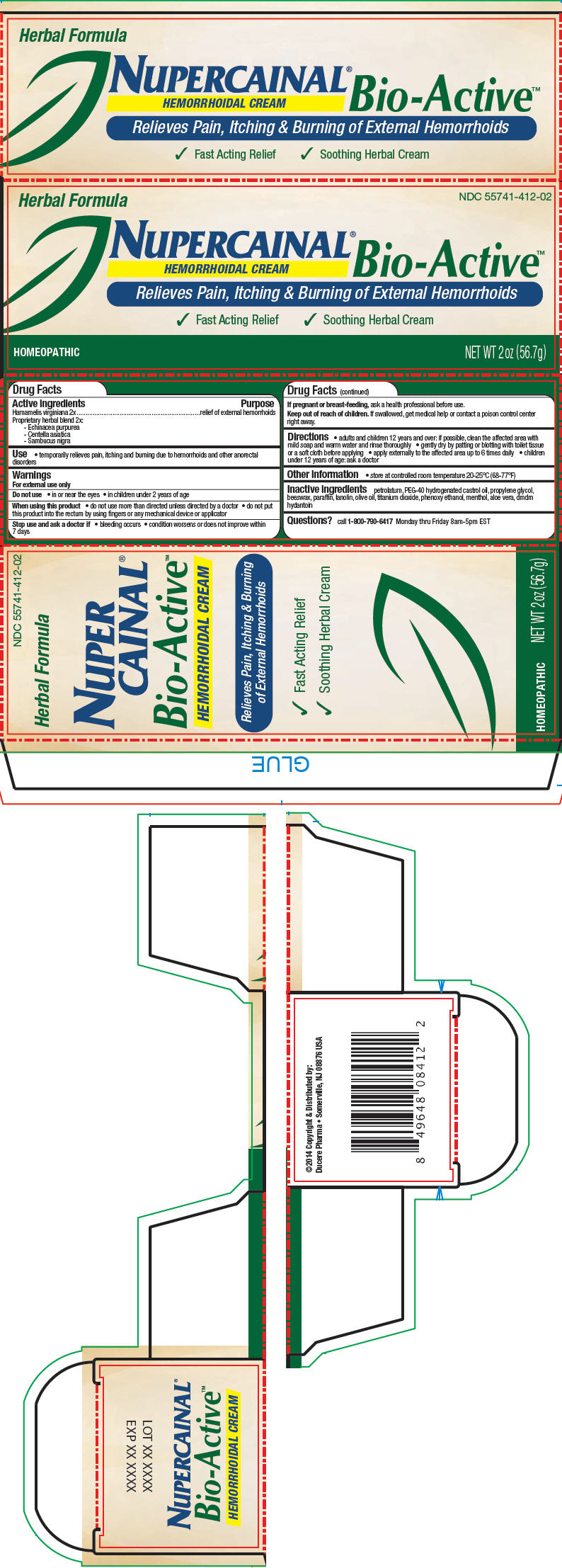
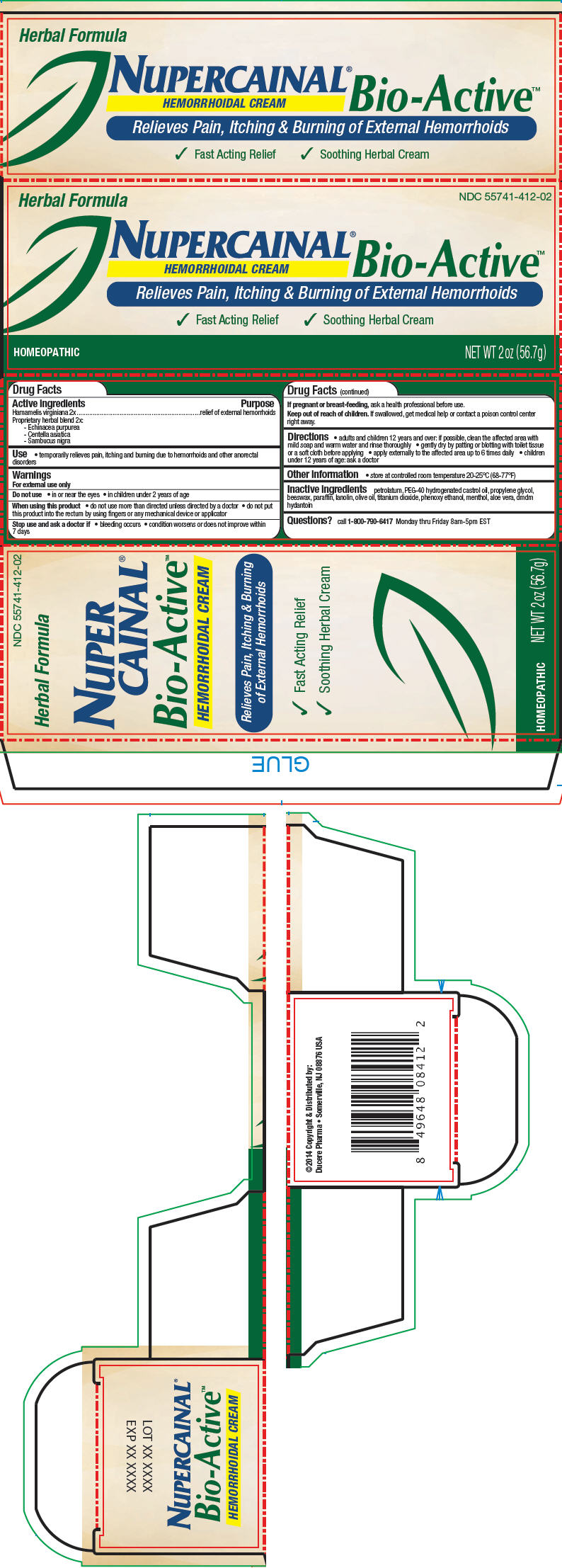
Label: NUPERCAINAL BIO-ACTIVE HEMORRHOIDAL- hamamelis virginiana root bark/stem bark, echinacea purpurea, centella asiatica, and sambucus nigra flowering top cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 55741-412-02 - Packager: Ducere Pharma
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 18, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions
- adults and children 12 years and over: if possible, clean the affected area with mild soap and warm water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- apply externally to the affected area up to 6 times daily
- children under 12 years of age: ask a doctor
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 56.7 g Tube Carton
-
INGREDIENTS AND APPEARANCE
NUPERCAINAL BIO-ACTIVE HEMORRHOIDAL
hamamelis virginiana root bark/stem bark, echinacea purpurea, centella asiatica, and sambucus nigra flowering top creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55741-412 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hamamelis Virginiana Root Bark/Stem Bark (UNII: T7S323PKJS) (Hamamelis virginiana root bark/stem bark - UNII:T7S323PKJS) Hamamelis Virginiana Root Bark/Stem Bark 5 g in 100 g Echinacea purpurea (UNII: QI7G114Y98) (Echinacea purpurea - UNII:QI7G114Y98) Echinacea purpurea 5 g in 100 g Centella asiatica (UNII: 7M867G6T1U) (Centella asiatica - UNII:7M867G6T1U) Centella asiatica 5 g in 100 g Sambucus Nigra Flowering Top (UNII: CT03BSA18U) (Sambucus nigra flowering top - UNII:CT03BSA18U) Sambucus Nigra Flowering Top 5 g in 100 g Inactive Ingredients Ingredient Name Strength petrolatum (UNII: 4T6H12BN9U) Polyoxyl 40 Hydrogenated Castor Oil (UNII: 7YC686GQ8F) propylene glycol (UNII: 6DC9Q167V3) yellow wax (UNII: 2ZA36H0S2V) paraffin (UNII: I9O0E3H2ZE) lanolin (UNII: 7EV65EAW6H) olive oil (UNII: 6UYK2W1W1E) titanium dioxide (UNII: 15FIX9V2JP) phenoxyethanol (UNII: HIE492ZZ3T) menthol (UNII: L7T10EIP3A) aloe vera leaf (UNII: ZY81Z83H0X) dmdm hydantoin (UNII: BYR0546TOW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55741-412-02 1 in 1 CARTON 1 56.7 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 04/07/2014 Labeler - Ducere Pharma (078715029)