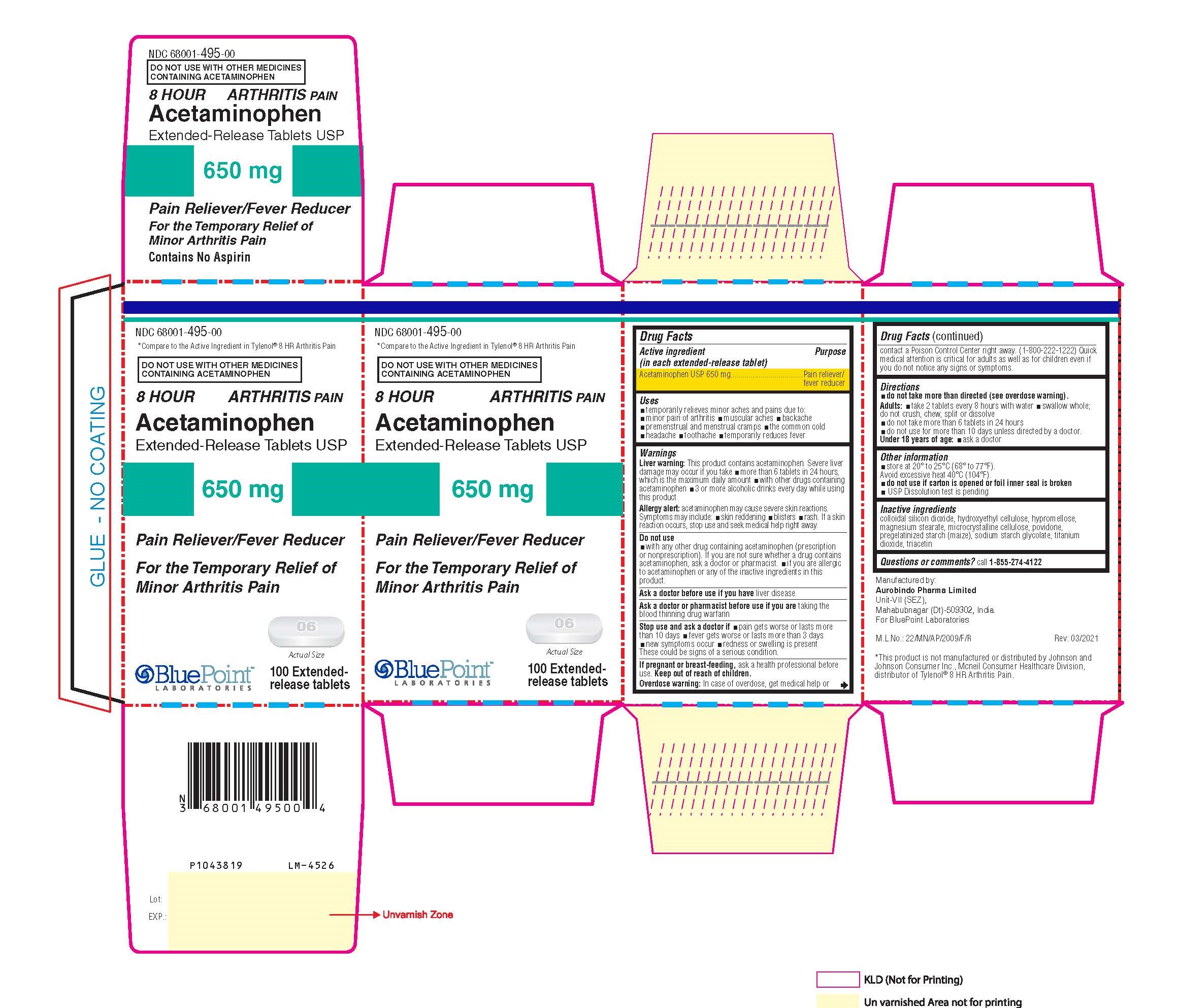
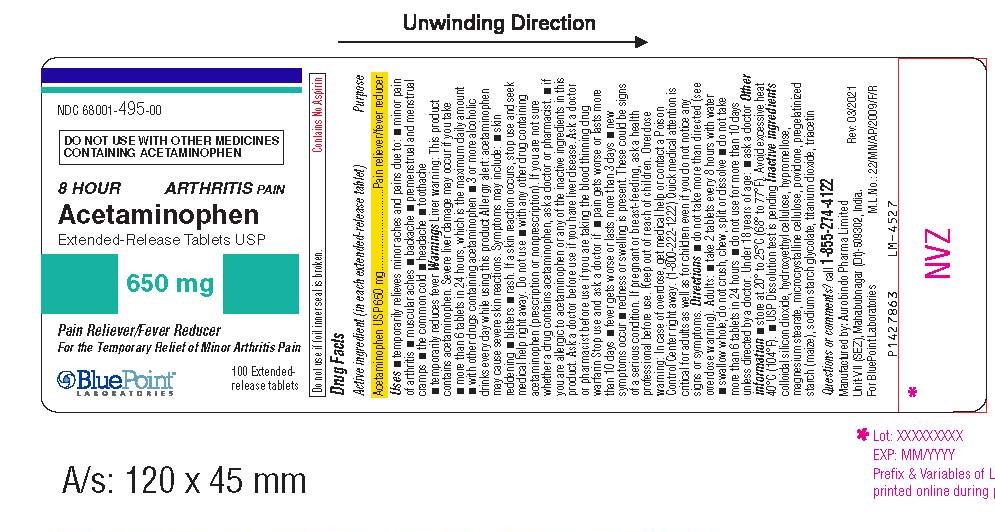
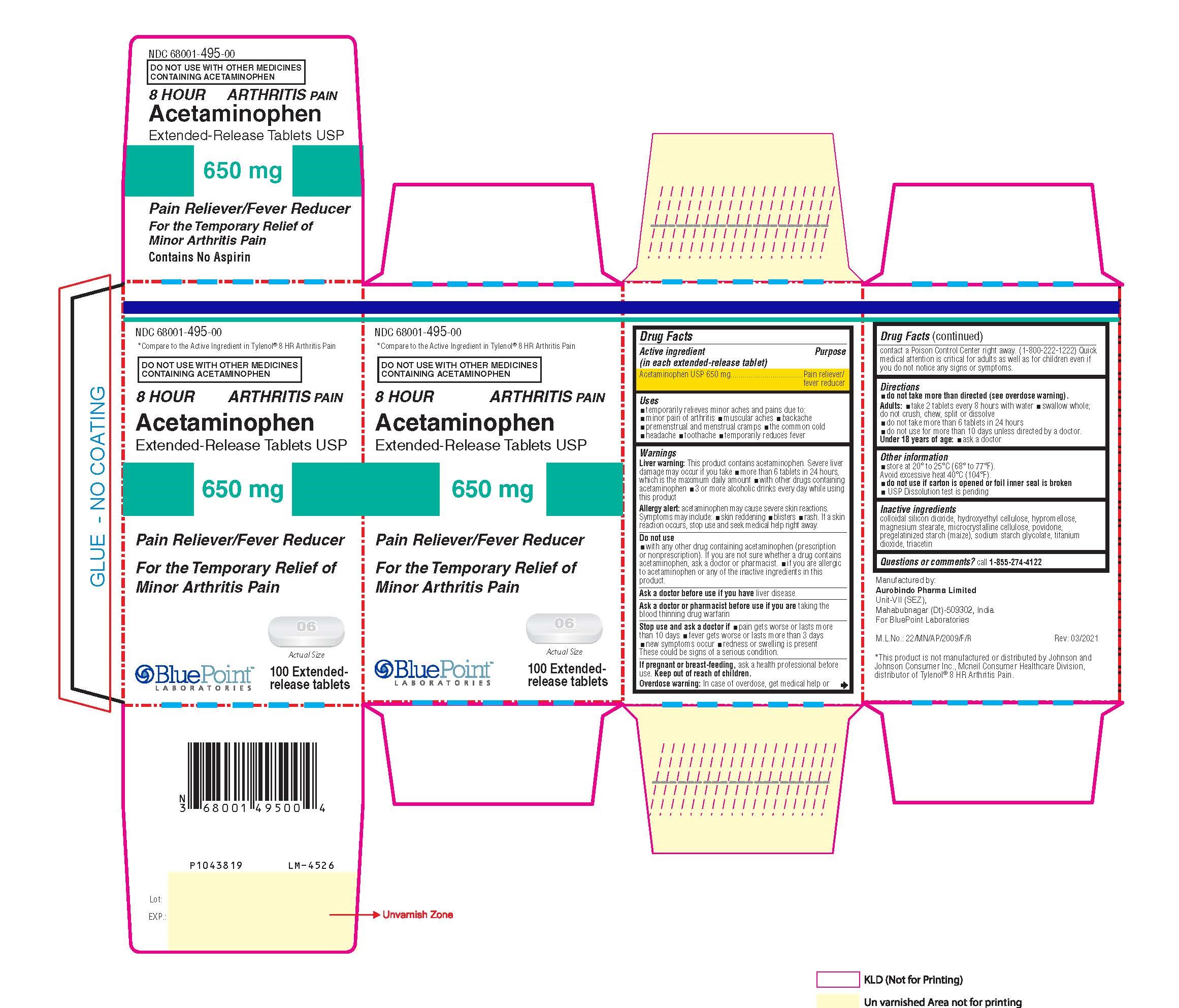
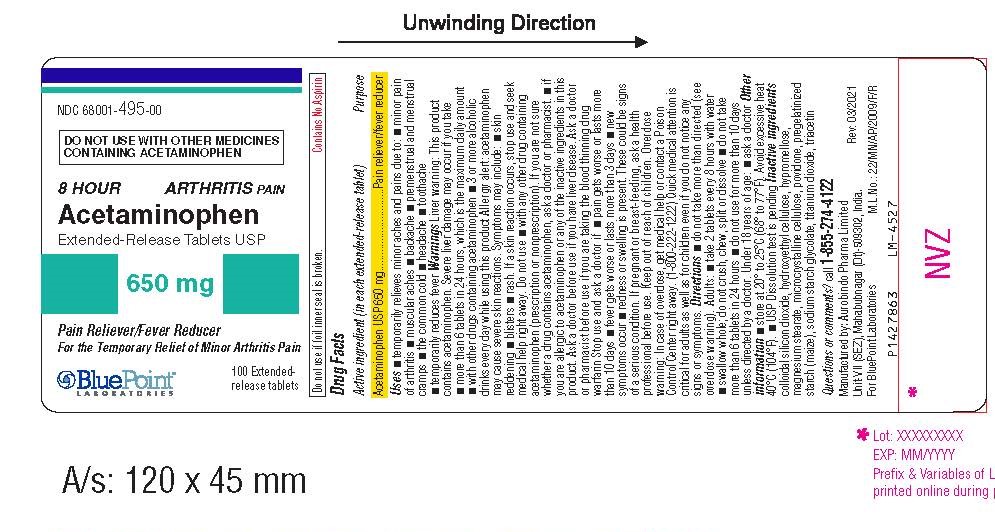
Label: ACETAMINOPHEN tablet
- NDC Code(s): 68001-495-00
- Packager: BluePoint Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient (in each extended release tablet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 6 tablets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
-
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure
whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product.
Ask a doctor before ue if you have liver disease.
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68001-495 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) HYDROXYETHYL CELLULOSE (140 MPA.S AT 5%) (UNII: 8136Y38GY5) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE B POTATO (UNII: 27NA468985) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white ((White to Off-White)) Score no score Shape CAPSULE ((Caplet)) Size 19mm Flavor Imprint Code I;06 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68001-495-00 1 in 1 CARTON 04/30/2021 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207229 04/30/2021 Labeler - BluePoint Laboratories (985523874) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Ltd 650381903 analysis(68001-495) , manufacture(68001-495)